Germline mutation analyses of malignant ground glass opacity nodules in non-smoking lung adenocarcinoma patients
- Published
- Accepted
- Received
- Academic Editor
- Ulrich Pfeffer
- Subject Areas
- Genetics, Molecular Biology, Oncology, Respiratory Medicine, Medical Genetics
- Keywords
- Lung adenocarcinoma, Germline mutation, Ground glass opacity, Non-smoking
- Copyright
- © 2021 Mao et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Germline mutation analyses of malignant ground glass opacity nodules in non-smoking lung adenocarcinoma patients. PeerJ 9:e12048 https://doi.org/10.7717/peerj.12048
Abstract
Background
Germline mutations play an important role in the pathogenesis of lung cancer. Nonetheless, research on malignant ground glass opacity (GGO) nodules is limited.
Methods
A total of 13 participants with malignant GGO nodules were recruited in this study. Peripheral blood was used for exome sequencing, and germline mutations were analyzed using InterVar. The whole exome sequencing dataset was analyzed using a filtering strategy. KOBAS 3.0 was used to analyze KEGG pathway to further identify possible deleterious mutations.
Results
There were seven potentially deleterious germline mutations. NM_001184790:exon8: c.C1070T in PARD3, NM_001170721:exon4:c.C392T in BCAR1 and NM_001127221:exon46: c.G6587A in CACNA1A were present in three cases each; rs756875895 frameshift in MAX, NM_005732: exon13:c.2165_2166insT in RAD50 and NM_001142316:exon2:c.G203C in LMO2, were present in two cases each; one variant was present in NOTCH3.
Conclusions
Our results expand the germline mutation spectrum in malignant GGO nodules. Importantly, these findings will potentially help screen the high-risk population, guide their health management, and contribute to their clinical treatment and determination of prognosis.
Introduction
Though therapeutic advances have been made using targeted therapy and immunotherapy, lung cancer continues to be the most common cause of cancer-related deaths worldwide (Siegel, Miller & Jemal, 2015). The majority of lung cancers are caused by somatic mutations that accumulate with age and germline mutations could explain a predisposition to cancer development.
Lung cancer is a complex disease that is mainly attributed to smoking (Hung et al., 2008). However, over 10% of lung cancer patients are non-smokers (Subramanian & Govindan, 2007). The development of lung cancer in never-smokers is associated with several potential risk factors, including environmental pollution and genetic predisposition (Malhotra et al., 2016). Germline mutations in lung cancer have been studied to some extent (Ikeda et al., 2014; Liu et al., 2016; Shukuya et al., 2018; Zhang et al., 2017), including some in familial settings (Kanwal et al., 2018; Tomoshige et al., 2015). There are also studies on non-smoking lung cancer cohorts (Donner et al., 2018; Renieri et al., 2014). Nonetheless, studies on germline mutations in lung cancer patients fall far short when compared to those on somatic mutations. There is a need to study germline mutations in lung cancer since they are related to the pharmacodynamics, prognosis, and interactions with somatic mutations (Bartsch et al., 2007; Erdem et al., 2012; Wang et al., 2018; Winther-Larsen et al., 2015).
GGOs observed on computed tomography are described as hazy areas but preserved broncho-vascular markings (Austin et al., 1996; Lee et al., 2014). Advances in high resolution computed tomography and its application in lung cancer screening have led to an increased detection rate of GGOs, with an estimated prevalence of 0.2–0.5% (Henschke et al., 2006). While many GGOs are benign and disappear with time, some are persistent and turn malignant. These tumours are frequently found in non-smokers and women lung cancer patients (Blons et al., 2006; Raz et al., 2006).
Here, we recruited a total of 13 non-smoking patients with malignant GGO nodules to study their germline mutations using whole exome sequencing (WES). The results provide a better understanding of molecular mechanisms underlying the development of GGOs and their predisposition to turn cancerous.
Materials & Methods
Study subjects
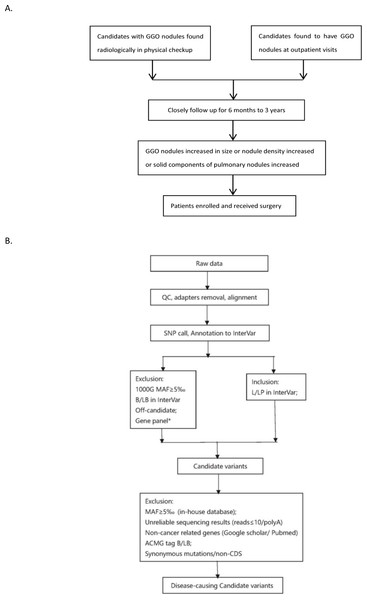
Candidates that were radiologically found to have small GGO nodules in physical checkup or who came to outpatients department for the reason of cough and checked by computed tomography to have small GGO nodules were closely followed up from 6 months to 3 years. When the GGO nodules increased in size or the nodule density increased or the solid components of pulmonary nodules increased, 13 patients were recruited and underwent surgery and thereafter they were histologically confirmed to have malignant GGOs in the Department of Cardiothoracic Surgery at Wuxi People’s Hospital affiliated to Nanjing Medical University, China, from April 1st, 2019 to August 30th, 2019. No other treatments were adopted. Written informed consent was obtained from all participants. Blood samples were collected before surgery and their clinical information was recorded. The research project was approved by the institutional review board of Wuxi People’s Hospital affiliated to Nanjing Medical University (no: HS2019014).
DNA extraction, library preparation, capture enrichment, and WES
Genomic DNA was extracted from peripheral blood collected from participants using a DNA blood mini kit (Qiagen, Germantown, MD, USA) following the manufacturer’s instructions. DNA concentration and purity were assessed by a Qubit fluorometer (Invitrogen, Carlsbad, CA, USA).
WES was conducted on 500 ng of genomic DNA from each participant. Fragment libraries were prepared from sheared samples by sonication, and exons were enriched by hybridisation capture with a SureSelect Human All Exon V6 Kit (Agilent, Santa Clara, CA, USA) according to the manufacturer’s protocol. Captured DNA was amplified followed by solid-phase bridge amplification. The paired-end library was sequenced on a NovaSeq 6000 platform (Illumina, San Diego, CA, USA). The data from this study were deposited in NCBI Sequence Read Archive under SRA accession: PRJNA613408.
Read alignment, variant calling, variant annotation, and filtering
Trimmomatic-0.36 (Bolger, Lohse & Usadel, 2014) was used as quality control for raw data and to remove adapters. Clean sequence reads were aligned to the human reference genome (GRCh37/b37 assembly) using Burrows-Wheeler Aligner software (version 0.7.10) (Li & Durbin, 2009). Picard (version 2.9.2, Broad Institute, Boston, MA, USA) was used to remove duplicates. Variant detection was performed using HaplotypeCaller in the Genome Analysis Toolkit 3.4 (https://gatk.broadinstitute.org/hc/en-us) (DePristo et al., 2011). Variants were annotated using InterVar database. Detailed stepwise filtering strategy for screening potential candidate germline mutations was described in Supplement 1.
KEGG pathway analysis
KEGG pathway analysis was conducted via KOBAS 3.0 (http://kobas.cbi.pku.edu.cn/).
Results
Clinical information of patients was summarised in Table 1. The mean age at onset of non-small cell lung cancer (NSCLC) in the cases was 61.5 years (range 48–79 years). All cases were non-smokers and 84.6% were females.
| Characteristics | ||
|---|---|---|
| Age at Diagnosis | Mean (SD) | 61.5 (8.7) |
| Range | 48–79 | |
| Gender | Male (%) | 2 (15.4) |
| Female (%) | 11 (84.6) | |
| Smoking history | Non-smokers (%) | 13 (100.0) |
| Smokers (%) | 0 (0) |
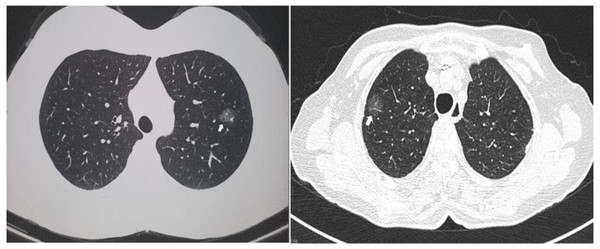
Two computed tomographic images are shown as representative of GGO nodules in the study cohort (Fig. 1). Of the 13 cases, 12 were diagnosed as lung adenocarcinomas while one was diagnosed as an atypical adenomatous hyperplasia. Five cases were adenocarcinoma in situ, four were invasive, and three were minimally invasive. Eight of the GGO nodules were located at the right upper lobe, two were at the right lower lobe, and three were at the left upper lobe. Detailed histologic information is presented in Table 2.
Figure 1: Representative of ground glass opacity nodules.
Two representative computed tomography images of ground glass opacity nodules. The arrows indicate the nodules.| Patient ID | Pathology | Tumour size (cm) | Tumour location |
|---|---|---|---|
| WL-1 | LUADa, invasive | 1.2 | right upper lobe |
| WL-2 | LUADa, AISb | 0.6 | left upper lobe |
| WL-3 | Atypical adenomatous hyperplasia | 0.5 | right upper lobe |
| WL-4 | LUADa, invasive | 1.5 | left upper lobe |
| WL-5 | LUADa, minimally invasive | 1.0 | right upper lobe |
| WL-6 | LUADa, minimally invasive | 0.6 | left upper lobe |
| WL-7 | LUADa, invasive | 2.0 | right upper lobe |
| WL-8 | LUADa, invasive | 2.0 | right lower lobe |
| WL-9 | LUADa, AISb | 0.8 | right upper lobe |
| WL-10 | LUADa, AISb | 0.7 | right upper lobe |
| WL-11 | LUADa, AISb | 0.6 | right lower lobe |
| WL-12 | LUADa, AISb | 0.8 | right upper lobe |
| WL-13 | LUADa, minimally invasive | 0.7 | right lower lobe |
Figure 2: Flowchart of analysis.
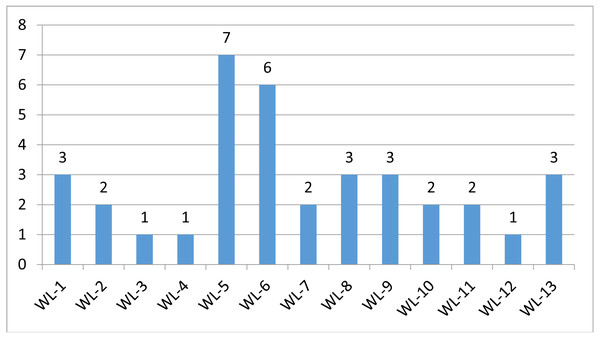
The stepwise filtering strategy used to screen for potential candidate germline mutations.We used a stepwise filtering strategy to screen for potential candidate variants (Fig. 2). Of 83,302 single-nucleotide variants (SNVs) located in exons of the whole exome, our filtering strategy identified 17 potential candidate variants (Table 3). Of the 17 candidate variants in 17 genes, NM_000700:exon6:c.A418T in AXAN1, NM_001184790:exon8:c.C1070T in PARD3, NM_001170721:exon4:c.C392T in BCAR1, NM_001127221:exon46:c.G6587A in CACNA1A, NM_001170634:exon5:c.G383A in FUS, and NM_002451:exon6:c.C538T in MTAP were present in three cases each. In addition, rs756875895 frameshift in MAX, NM_001199292:exon7:c.C482G in HSD17B4, NM_005732:exon13:c.2165_2166insT in RAD50, NM_001350128:exon11:c.T1172C in PPOX, NM_001098816:exon28:c.A4751G in TENM4, NM_004004:exon2:c.235delC in GJB2, and NM_001142316:exon2:c.G203C in LMO2 were present in two cases each. The remaining variants were present in one case each. Of the 17 variants, 12 were nonsynonymous mutations, four were frameshift deletions, and one was a stopgain. The distribution of mutations in each patient was shown in Fig. 3.
| Gene | Position | RS | Ref/Alt | Protein alteration | Genetic model | Type of mutation | InterVar annotation | VAF in patients | VAF in GnomAD_EAS | No. of patients with mutation |
|---|---|---|---|---|---|---|---|---|---|---|
| ANXA1 | Chr 9: 75775752 | – | A/T | NM_000700:exon6:c.A418T:p.I140F | – | nonsynonymous SNV | Uncertain significance | 0.0833 | – | 3 |
| NOTCH3 | Chr 19: 15281611 | – | T/G | NM_001184790:exon8:c.C1070T:p.T357M | ADa nonsynonymous SNV | Likely pathogenic | 0.0278 | – | 1 | |
| PARD3 | Chr 10: 34671665 | rs116642073 | G/A | NM_001184790:exon8:c.C1070T:p.T357M | – | nonsynonymous SNV | Uncertain significance | 0.0833 | 0 | 3 |
| BCAR1 | Chr 16: 75269775 | rs1047683608 | G/A | NM_001170721:exon4:c.C392T:p.P131L | – | nonsynonymous SNV | Uncertain significance | 0.0833 | 0 | 3 |
| CYP21A2 | Chr 6: 32008198 | rs7755898 | C/T | NM_001128590:exon7:c.C865T:p.Q289X | – | Stopgain | Likely pathogenic | 0.0278 | 0.0001 | 1 |
| LDLRAP1 | Chr 1: 25870253 | – | G/- | NM_015627:exon1:c.65delG:p.W22fs | ARb frameshift deletion | Pathogenic | 0.0278 | – | 1 | |
| CACNA1A | Chr 19: 13319766 | rs373192655 | C/T | NM_001127221:exon46:c.G6587A:p.R2196Q | AD | nonsynonymous SNV | Uncertain significance | 0.0556 | 0.0015 | 3 |
| MAX | Chr 14: 65551007 | rs756875895 | G/- | NM_001271068:exon3:c.154delC:p.L52fs | AD | Frameshift deletion | Likely pathogenic | 0.0278 | – | 2 |
| HSD17B4 | Chr 5: 118814630 | rs763363391 | C/G | NM_001199292:exon7:c.C482G:p.A161G | AR | nonsynonymous SNV | Likely pathogenic | 0.0556 | 0.0002 | 2 |
| RAD50 | Chr 5: 131931460 | rs587781454 | -/T | NM_005732:exon13:c.2165_2166insT:p.K722fs | – | Frameshift deletion | Uncertain significance | 0.0556 | – | 2 |
| PPOX | Chr 1: 161140719 | – | T/C | NM_001350128:exon11:c.T1172C:p.L391P | AD | nonsynonymous SNV | Likely pathogenic | 0.0278 | – | 2 |
| FUS | Chr 16: 31195580 | – | G/A | NM_001170634:exon5:c.G383A:p.S128N | AD | nonsynonymous SNV | Uncertain significance | 0.0556 | – | 3 |
| MTAP | Chr 9: 21854717 | rs891972796 | C/T | NM_002451:exon6:c.C538T:p.R180W | AD | nonsynonymous SNV | Uncertain significance | 0.0556 | 0 | 3 |
| TENM4 | Chr 11: 78412907 | – | T/C | NM_001098816:exon28:c.A4751G:p.Q1584R | AD | nonsynonymous SNV | Likely pathogenic | 0.0833 | – | 2 |
| GJB2 | Chr 13: 20763485 | rs80338943 | G/- | NM_004004:exon2:c.235delC:p.L79fs | AD | Frameshift deletion | Uncertain significance | 0.0556 | – | 2 |
| TTN | Chr 2: 179427779 | rs192360370 | G/A | NM_003319:exon154:c.C55885T:p.R18629C | AR/AD | nonsynonymous SNV | Uncertain significance | 0.0278 | 0.0038 | 1 |
| LMO2 | Chr 11: 33886202 | – | C/G | NM_001142316:exon2:c.G203C:p.G68A | nonsynonymous SNV | Uncertain significance | 0.0556 | – | 2 |
We identified four potential deleterious frameshift deletions: rs80338943 in GJB2, rs587781454 in RAD50, rs756875895 in MAX, each occurring in two cases, and a frameshift in LDLRAP1, occurring in one case. The first frameshift, rs80338943 in GJB2, causing a p.L79fs fusion, was annotated as uncertain significance by InterVar. The second frameshift, rs587781454 in RAD50 caused a p.K722fs fusion and was annotated as pathogenic from InterVar. The third frameshift, rs756875895 in MAX, was annotated as likely pathogenic in InterVar, causing a fusion of p.L52fs. The frameshift in LDLRAP1 caused a fusion of p.W22fs in one case and was interpreted as pathogenic from InterVar, it might be deleterious in the development of lung cancer. Another potential deleterious variant was a stopgain, rs7755898 in CYP21A2, causing a protein change of p.Q289X which was likely pathogenic according to InterVar.
The other interesting candidates were four likely pathogenic SNVs annotated from InterVar: NOTCH 3:p.T357M (present in one case), HSD17B4 p.A161G (present in two cases), PPOX p.L391P (present in two cases), and TENM4 p.Q1584R (present in two cases).
There were five SNVs annotated as uncertain significance by InterVar that were present in three patients: ANXA1:p.I140 F, BCAR1:p.P131L, CACNA1A:p.R2196Q, FUS:p.S128N, and MTAP:p.R180W.
There were two additional candidate variants, LMO2 p.G68A and TTN p.R18629C, that were present in two cases and one case, respectively (Table 3). Their annotations by InterVar were of uncertain significance.
KEGG analysis did not indicate pathways that were related to AXAN1, TENM4 and GJB2. Pathways of BCAR1, CYP21A2, LPLRAP1, HSD17B4, MTAP, PPOX and TTN were not associated with cancer. Pathways derived from NOTCH3, PARD3, CACNA1A, MAX, RAD50, FUS and LMO2 were cancer-related. The details were shown in a (Table S). Mutations in these genes were considered unlikely to cause cancer, therefore they would not be discussed here.
Figure 3: Mutation distriubtion.
The distribution of germline mutations in each patient.Discussion
Although there are studies available on genetic mutations of lung cancer, the heritability of lung cancer, especially for GGO nodules, remains understudied compared to sporadic lung cancer. Using WES, our study reports germline mutations in GGO nodules of non-smoker lung cancer patients, largely females.
The discovery of germline mutations is very significant for both basic research and clinical treatment of lung cancer. First, germline mutations may play a role in tumorigenesis. Wang et al. (2018) reported that germline mutations interacted with somatic mutations, indicating their role in lung tumorigenesis. Tomoshige et al. (2015) also reported that germline mutations could cause familial lung cancer. Second, germline mutations are valuable for prognosis (Erdem et al., 2012). For example, a study by Winther-Larsen et al. (2015) found that genetic polymorphism in the epidermal growth factor receptor could predict the outcome in advanced NSCLC patients treated with erlotinib. Third, germline mutations are closely associated with a genetic predisposition to cancer, and screening for germline mutations is beneficial to the susceptible population (Chen et al., 2015) and for their health management.
In this study, we used a highly selective population, lung adenocarcinoma patients with GGOs, to investigate germline mutations and their possible role in the predisposition to lung cancer. In our cohort, 11 of 13 were females and all were non-smokers. The ethnicity of all patients was Han Chinese. The aforementioned facts were consolidated with the notion that malignant GGO nodules occur frequently in non-smokers and women (Blons et al., 2006; Raz et al., 2006).
Strong evidence for two deleterious germline mutations (rs587781454 in RAD50 and rs756875895 in MAX) has been shown in lung cancer patients. rs587781454 in RAD50 was reported as a hereditary predisposition and labelled as pathogenic in ClinVar (Nykamp et al., 2017). rs756875895 in MAX was labelled as likely pathogenic by InterVar. Both variants occurred simultaneously in two females (WL-5 and WL-6). Both had minimally invasive GGO nodules. How these mutations in the same patient affected lung tumorigenesis is worth examining.
There was one likely pathogenic variant in NOTCH 3 (WL-13). The expression of NOTCH 3 was inversely associated with the sensitivity to platinum-based chemotherapy in patients with NSCLC. The NOTCH 3 protein, rather than the gene polymorphism, is associated with the chemotherapy response and prognosis of advanced NSCLC patients (Shi et al., 2014).
Though annotated as uncertain significance by InterVar, three patients carried variants in BCAR1 (WL-7, WL-10 and WL-13) and CACNA1A (WL-5, WL-6 and WL-9). Increased expression of BCAR1 was associated with poor prognosis and carcinogenesis in NSCLC (Deng et al., 2013; Huang et al., 2012). Overexpression of CACNA1A predicted a poor prognosis in NSCLC (Zhou et al., 2017). There were one additional candidate variants, LMO2 p.G68A in WL-1 and WL-8. Collectively, these findings suggest that germline mutations may function by regulating gene expression and thereby affect cancer development and/or prognosis.
Our study has limitations. First, the sample size is small. In our study, only non-smoker patients with malignant GGOs were enrolled. Second, gene expression was not investigated. Finally, the identified germline mutations have not been validated. These limitations restrict conclusions about their causative effects on tumorigenesis and their roles as biomarkers for prognosis or for treatment response.
Conclusions
In summary, our results demonstrate potentially deleterious germline mutations in GGO nodules in non-smoking lung adenocarcinoma patients. These findings significantly expand the spectrum of genetic variants that may affect the response to therapies and patient survival and possibly increase the risk of being germline mutation carriers. However, due to the small patient samples, our observations encourage further studies. In future, prospective studies, expanding enrolled patients and functional studies should be performed to better understand their causative roles in tumorigenesis and prognosis, and to better manage patients’ health.
Supplemental Information
Methods used to screen candidate variants
Detailed description of methods used to screen candidate variants
CT images and IHC
Computed tomography and immunohistochemistry images of patients