Comparative studies of two AA10 family lytic polysaccharide monooxygenases from Bacillus thuringiensis
- Published
- Accepted
- Received
- Academic Editor
- Rogerio Sotelo-Mundo
- Subject Areas
- Biochemistry, Biotechnology
- Keywords
- Lytic polysaccharide monooxygenase, Synergy, CBM5, Chitin, Bacillus thuringiensis
- Copyright
- © 2022 Zhang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Comparative studies of two AA10 family lytic polysaccharide monooxygenases from Bacillus thuringiensis. PeerJ 11:e14670 https://doi.org/10.7717/peerj.14670
Abstract
Bacillus thuringiensis, known to be one of the most important biocontrol microorganisms, contains three AA10 family lytic polysaccharide monooxygenases (LPMOs) in its genome. In previous reports, two of them, BtLPMO10A and BtLPMO10B, have been preliminarily characterized. However, some important biochemical features and substrate preference, as well as their potential applications in chitin degradation, still deserve further investigation. Results from present study showed that both BtLPMO10A and BtLPMO10B exhibit similar catalytic domains as well as highly conserved substrate-binding planes. However, unlike BtLPMO10A, which has comparable binding ability to both crystalline and amorphous form of chitins, BtLPMO10B exhibited much stronger binding ability to colloidal chitin, which mainly attribute to its carbohydrate-binding module-5 (CBM5). Interestingly, the relative high binding ability of BtLPMO10B to colloidal chitin does not lead to high catalytic activity of the enzyme. In contrast, the enzyme exhibited higher activity on β-chitin. Further experiments showed that the binding of BtLPMO10B to colloidal chitin was mainly non-productive, indicating a complicated role for CBM5 in LPMO activity. Furthermore, synergistic experiments demonstrated that both LPMOs boosted the activity of the chitinase, and the higher efficiency of BtLPMO10A can be overridden by BtLPMO10B.
Introduction
Chitin can be considered as the second most abundant biopolymer on Earth. It consists of β-1,4-linked N-acetylglucosamine and is widely distributed in the exoskeleton of crustaceans and in the cell walls of insects and fungi (Tharanathan & Kittur, 2003). Chitin can be classified into α (anti-parallel chains) and β (parallel chains) crystalline forms. Chitin is responsible for providing important characteristics such as rigidity and strength to the cell wall, and it also adds to the defense of the cell against pathogens and various predators (Beckerman et al., 2013; Bowman & Free, 2006; Brunner et al., 2009; Vincent & Wegst, 2004). Thus, the destruction of the crystalline structure of chitin in insects or fungi with the application of chitin-degrading microorganisms has been thought to be a broad-spectrum biocontrol strategy in agriculture (Le & Yang, 2019). Besides, the conversion of chitin waste, such as shrimp and crab shells, into chitooligosaccharides contributes to increased nutritional benefits in the food industry (Le & Yang, 2019).
In nature, chitin-degrading organisms have evolved a series of enzymes that are involved in the synergetic depolymerization of chitin, including glycoside hydrolases (GHs) and lytic polysaccharide monooxygenases (LPMOs) (Vaaje-Kolstad et al., 2013; Vaaje-Kolstad et al., 2010). GHs typically have their activity targeted on the amorphous region (non-processive enzymes) or the end of the chitin chains (processive enzymes) and cleave the glycosidic bond using a hydrolytic mechanism (Vaaje-Kolstad et al., 2013). On the other hand, LPMOs are recently discovered as copper-dependent metallo-enzymes which can oxidatively destroy the crystalline region of the recalcitrant polysaccharides and are also responsible for boosting the efficiency of GHs (Merino & Cherry, 2007; Vaaje-Kolstad et al., 2010). Due to their promising application in biomass bioconversion and biorefinery, LPMOs have been given special attention (Johansen Katja, 2016; Martínez et al., 2017; Monclaro & Filho, 2017). In addition, some researchers have proposed that LPMOs may also be associated with the virulence of the pathogens (Agostoni, Hangasky & Marletta, 2017; Paspaliari et al., 2015; Sabbadin et al., 2021; Wong et al., 2012).
Bacillus thuringiensis is one of the most important biocontrol microorganisms, which has been used in agriculture as a biopesticide for a long time to control various invertebrate species (Melo, Soccol & Soccol, 2014). Besides the cytotoxicity of organic insecticides, B. thuringiensis secretes diverse chitin-degrading enzymes, including LPMOs, which could affect insect growth, and ultimately lead to death of insects (Veliz, Martínez-Hidalgo & Hirsch, 2017; Kramer & Muthukrishnan, 1997; Melo, Soccol & Soccol, 2014). In the genome of B. thuringiensis, three AA10 family LPMOs encoding genes can be discovered, among which two had already been characterized (Manjeet et al., 2019; Zhang et al., 2015). BtLPMO10A is a single-modular LPMO which acts on both crystalline chitin (α- and β-chitin) and colloidal chitin, and generates products with different patterns (Zhang et al., 2015). In contrast, BtLPMO10B (reported by Manjeet et al. (2019) and denoted as BtLPMO10A-FL) is a multi-modular LPMO and the roles of individual domains in substrate (crystalline chitin) binding have been characterized. However, biochemical features such as the effect of substrate binding on H2O2 generation, as well as their synergistic activity with chitinase in chitin degradation still worth further investigation. In this study, the biochemical features and substrate preference of BtLPMO10A and BtLPMO10B were compared with the aim of identifying and understanding their functions in chitin degradation. The obtained results showed the significantly different substrate preferences of the two enzymes although they shared highly conserved substrate binding surfaces in their catalytic domain. The C-terminal domains of BtLPMO10B do enhance the substrate binding ability of the enzyme, especially on colloidal chitin, whereas it has little effect on the activity of BtLPMO10B. Synergetic assays indicated that the efficiency of chitinase can be significantly improved by both the BtLPMO10A and the BtLPMO10B, whereas the higher effect of the BtLPMO10A can be attenuated by BtLPMO10B.
Material and Methods
Sequence and structure analysis
The sequences of BtLPMO10A (GenBank ID: AJP62637) and BtLPMO10B (GenBank ID: ALP73598) can be accessed in the Genbank database and the crystal structure of BtLPMO10A was obtained from the Protein Data Bank database with accession code 5WSZ (Zhang et al., 2020). The three-dimensional structure of the catalytic domain of BtLPMO10B (BtLPMO10B-CD) was generated by homology modeling using Modeller 9.19 (Webb & Sali, 2016) with the crystal structure of BaLPMO10A from Bacillus amyloliquefaciens (PDB ID: 2YOX) (Hemsworth et al., 2013) as the template since they share the highest sequence identity (66%) (Zhang & Madden, 1997). After been further validated by DOPE score, a structure-based sequence alignment of BtLPMO10A and BtLPMO10B was conducted using Mega 7.0 (Kumar, Stecher & Tamura, 2016) and ESPript 3.0 (Robert & Gouet, 2014).
Cloning of BtLPMO10B and its catalytic domain BtLPMO10B-CD
We produced three recombinant LPMOs from B. thuringiensis kurstaki ACCC10066 in E. coli BL21 (DE3), including the previously reported BtLPMO10A which stored in the lab. The gene encoding BtLPMO10B was amplified from the genomic DNA of B. thuringiensis kurstaki ACCC10066 using a forward primer F1: 5′-GGAATTCCATATGCACGGTTTTGTTGAAAAGCCCGGTA-3′ encoding a restriction site for NdeI and a reverse primer R1: 5′-CCGCTCGAGCACTGTTTTCCATAATGATAATGCA- 3′ with a restriction site for XhoI. The amplified gene was then subcloned into the pET23b vector through double digestion with the two restriction enzymes. The catalytic domain of BtLPMO10B (BtLPMO10B-CD) was synthesized and cloned into the same vector by the Taihe Biotechnology Co., Ltd (Beijing, China). After verification by sequencing, three recombinant plasmids were transformed into Escherichia coli BL21 (DE3) competent cells, respectively, for protein expression.
Protein expression and purification
The recombinant E. coli BL21 (DE3) cells were cultivated in 1 L Luria-Bertani (LB) medium at 37 °C with constant shaking at the speed of 180 rpm. When the OD600 of the culture reached 0.6, a final concentration of 0.05 mM IPTG and 0.2 mM CuSO4 were added and the cultivation was continued for an additional 4 h at 30 °C. Afterword, the cells were harvest by centrifugation at 4 °C for 10 min with the speed of 8,000 × g, and then resuspended in 100 mL of hypertonic solution containing 100 mM Tris–HCl pH 8.0, 20% sucrose and 0.5 mM EDTA. This step was performed two times. Finally, the precipitated cells obtained by centrifugation were resuspended in 100 mL hypotonic solution (1 mM MgCl2) and incubated on ice for 10 min. After 10 min of centrifugation at 8,000 × g, the supernatant was collected for further purification.
For the purification of BtLPMO10A, a chitin beads affinity chromatography method was performed as described previously (Zhang et al., 2015). For the BtLPMO10B, a similar method was adopted with some modifications. The loading buffer was changed to 20 mM Tris–HCl (pH 8.0) and 0.15M (NH4)2SO4, and the protein was eluted by 20 mM acetic acid. For the purification of BtLPMO10B-CD, an ion exchange chromatography with HiTrap Q column (GE Healthcare, USA) was performed. The protein solution was loaded onto the column equilibrated with 20 mM Tris–HCl buffer (pH 7.5) and eluted with a linear salt gradient using 1 M NaCl (pH 7.5). The obtained fractions were pooled and concentrated using the Amicon 8400 stirred cell (Millipore, Burlington, MA, USA) installed with a 3kDa cut-off membrane. Samples purity was analyzed by SDS-PAGE and the protein concentrations were measured by Bradford, using bovine serum albumin as a standard.
Substrate binding assays
The reactions were conducted in 20 mM Tris–HCl (pH 8.0) buffer containing 1 µM enzyme and 5 mg mL−1 α-chitin, β-chitin and colloidal chitin, respectively, prepared according to the procedure described previously (Zhang et al., 2015). The mixture was incubated 6 h at 25 °C with constant shaking at 800rpm using Thermo block (Eppendorf, Hamburg, Germany). After been separated from the mixture by filtration through a 0.22 µm membrane, the concentrations of the free proteins measured using the Quick Start™ Bradford assay (Bio-Rad, Hercules, CA, USA). The mixtures without substrate were treated in the same way and used as the basis for calculating the percentage of free and bound protein.
H2O2 generation assays
The reactions were conducted in 20 mM Tris–HCl (pH 8.0) buffer containing 1 µM enzyme, 1 mM ascorbic acid and 5 mg mL−1 α-chitin, β-chitin and colloidal chitin, respectively. The mixture was incubated 2 h at 30 °C with constant shaking at 800 rpm using Thermo block (Eppendorf, USA). After been separated from the reaction mixture by filtration through a 0.22 µm membrane, the concentrations of H2O2 in the supernatant were measured using the Fluorimetric Hydrogen Peroxide Assay Kit (Sigma, St. Louis, MO, USA). The reactions without substrate were set as the control.
Enzymatic reactions
Enzymatic reaction was performed in a 500 µL reaction mixture containing 5 mg mL−1 substrate, 20 mM Tris–HCl (pH 8.0), 1 µM enzyme, and 1 mM ascorbic acid. For the reaction using both BtLPMO10A and BtLPMO10B, 0.5 µM BtLPMO10A and BtLPMO10B was added. The reaction was last for 16 h at 30 °C with constant shaking at the speed of 800 rpm. After been separated from the reaction mixture by filtration through a 0.22 µm membrane, the generated oligosaccharides were analyzed using matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF MS) and high-performance liquid chromatography (HPLC) with protocols described previously (Zhang et al., 2015; Zhang et al., 2020). Since the content of produced DP4ox is a feasible parameter to evaluate the efficiency of LPMO reactions (Zhang et al., 2020), the peak areas of DP4ox, DP5ox, and DP6ox were calculated for comparative analysis.
Synergetic assays
The chitinase synergetic experiments were carried out in 20 mM PBS buffer (pH 6.0) containing 5 mg mL−1 α-chitin (Sigma, USA), 1 µM SmChiB (anexo-type chitinase from Serratia marcescens), 1 µM LPMO (BtLPMO10A or BtLPMO10B) and 1 mM ascorbic acid mixed in 500 µL reaction. The mixtures were incubated at 30 °C for 4, 8, 12, 24, 48, 72 h with an 800 rpm shaking. After separated the products from the reaction mixture by filtration through a 0.22 µm membrane, an equal volume of acetonitrile was added into the product solution. For BtLPMO10A and BtLPMO10B synergy studies, 0.5 µM BtLPMO10A and BtLPMO10B was added into the mixture. The reaction without the presence of LPMO was used as the control. The (GlcNAc)2 released from the reactions was analyzed by HPLC equipped with an X-Amide column and a UV detector at 195 nm. The concentration of (GlcNAc)2 in samples was calculated using commercial (GlcNAc)2 as a standard. All experiments were performed in triplicates.
Results
Structure and sequence analysis of BtLPMO10A and BtLPMO10B
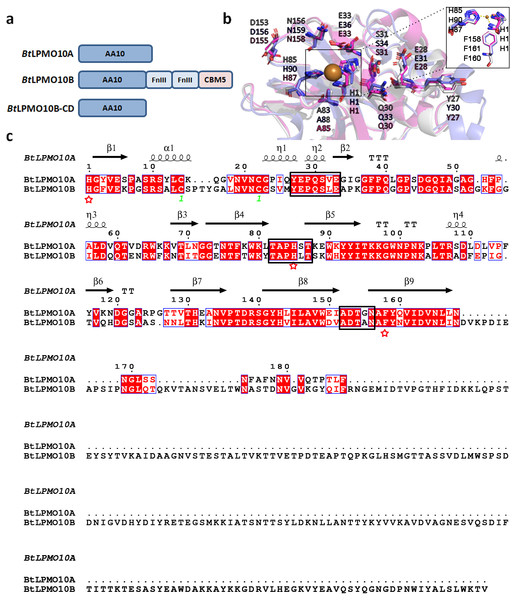
BtLPMO10A and BtLPMO10B shares a sequence identity of 61%, and both enzymes contain a typical active site of AA10 family LPMOs that are comprised of a type II copper ion coordinated with two fully conserved histidines (Figs. 1A and 1B). A Phenylalanine residue was identified to be the axial residue of the copper ion. Furthermore, the residues in the substrate binding surface, as shown in Fig. 1B, were also highly conserved, which may involve in substrate binding similar as described in Sm CBP21A (Vaaje-Kolstad et al., 2005). Structure-based sequence alignment indicated that BtLPMO10A and BtLPMO10B possess similar loop regions surrounding the catalytic center (Fig. 1C).
Figure 1: Structure and sequence comparison of BtLPMO10A and BtLPMO10B.
(A) Domain structure of BtLPMO10A, BtLPMO10B and BtLPMO10B-CD(1 to 178 amino acids in BtLPMO10B). (B) Close-up view of the substrate-binding surfaces of SmCBP21A (purple), BtLPMO10A (gray) and BtLPMO10B-CD (blue) which was modeled using BaLPMO10A (PDB ID: 2YOX) as template. The residues putative interacting with chitin is shown. (C) Structure-based sequence alignment of BtLPMO10A and BtLPMO10B. The amino acids from different loop regions surrounding the catalytic center were highlighted in the black rectangle and the residuescoordinated with the copper ion are marked by red stars.Substrate binding ability and activity of BtLPMO10A and BtLPMO10B
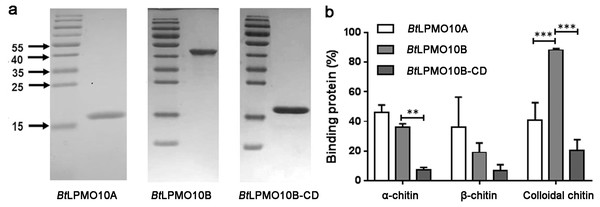
The substrate-binding ability of BtLPMO10A and BtLPMO10B on α-, β-chitin, and colloidal chitin were assessed. As shown in Fig. 2B, BtLPMO10A exhibits comparable binding ability to all three types of chitins. In contrast, for BtLPMO10B, 80% of the enzyme protein was found bound to the colloidal chitin, which is significantly higher than the percentages of protein bound to the crystalline α- and β-chitin. Deletion of the extra domains of BtLPMO10B significantly decreased its binding ability towards all three kinds of chitins tested (Fig. 2B).
Figure 2: Substrate binding ability and activity of BtLPMO10A, BtLPMO10B and BtLPMO10B-N.
(A) Determination of the purity of BtLPMO10B and BtLPMO10B-CD by SDS-PAGE. (B) Substrate binding ability of BtLPMO10A,BtLPMO10B, and BtLPMO10B-CD towards different type of chitin. The proportion of the bound proteins was calculated based on the residual proteins in the supernatant.Product analysis of BtLPMO10A, BtLPMO10B, and BtLPMO10B-CD
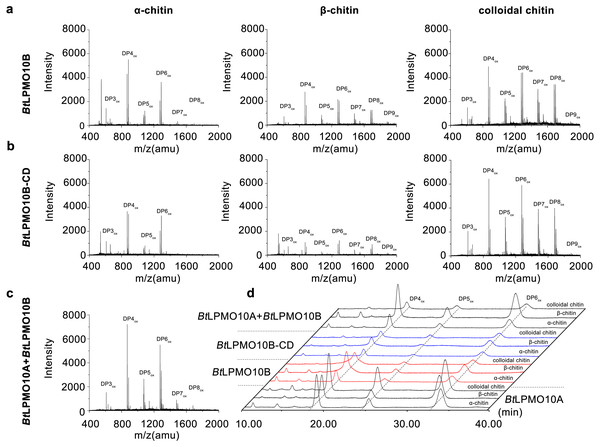
The enzymatic activity of BtLPMO10B towards various types of chitins, including α-, β-chitin, colloidal chitin, and chitin oligosaccharides were investigated using MALDI-TOF MS. As shown in Figs. 3A and 3B, BtLPMO10B and BtLPMO10B-CD can act on α-, β-chitin, and colloidal chitin and generate a product profile with even-numbered oxidized oligosaccharides as the dominant products. The products of BtLPMO10A, BtLPMO10B and BtLPMO10B-CD from different kinds of chitins were further analyzed by HPLC (Table 1, Fig. 3D). The results showed that the DP4ox and DP6ox were the main oxidation products for BtLPMO10A alone or in combination with BtLPMO10B on almost all tested chitins. Differently, the DP4ox was the predominant product for BtLPMO10B reactions and the highest production of the DP4ox was obtained when β-chitin was used as the substrate. The deletion of the CBM5 from BtLPMO10B significantly reduced the production of DP4ox, while relative mild reduction of observed for DP5ox and DP6 ox (Table 1). Moreover, although the binding ability of BtLPMO10B-CD toward colloidal chitin was significantly lower than the full-length enzyme (Fig. 2B), the amount of oxidized products (DP4ox, DP5ox, and DP6ox) generated by BtLPMO10B-CD were similar as compared to BtLPMO10B. The combination of BtLPMO10A and BtLPMO10B led to similar product profiles on both α-chitin and colloidal chitin as compared to BtLPMO10B. In contrast, the product profile on β-chitin with the combined BtLPMO10A and BtLPMO10B was similar to that of BtLPMO10A. These results suggest that BtLPMO10B can affect the activity of BtLPMO10A on α-chitin and colloidal chitin.
Figure 3: Product analysis of BtLPMO10A, BtLPMO10B, and BtLPMO10B-CD.
The products of LPMOs towards α-chitin, β-chitin and Colloidal chitin analyzed by MALDI-TOF MS (A, B, and C) or HPLC (D). The reactions were performed using 1 µM of enzymes and 1 mM of ascorbic acid.| Chitin forms | Enzyme combinations | Area (µV*s) | ||
|---|---|---|---|---|
| DP4ox | DP5ox | DP6ox | ||
| α-Chitin | BtLPMO10A+BtLPMO10B | 2003029 | 820042 | 2231064 |
| BtLPMO10B-CD | 1202074 | 427881 | 1292101 | |
| BtLPMO10B | 2145732 | 412200 | 1016363 | |
| BtLPMO10A | 5064803 | 1872054 | 5236980 | |
| – | 0 | 0 | 0 | |
| β- Chitin | BtLPMO10A+BtLPMO10B | 4229223 | 1652738 | 5399536 |
| BtLPMO10B-CD | 1592714 | 349987 | 1302021 | |
| BtLPMO10B | 4776729 | 799804 | 1920114 | |
| BtLPMO10A | 2805547 | 1382478 | 5238538 | |
| – | 0 | 0 | 0 | |
| Colloidal chitin | BtLPMO10A+BtLPMO10B | 1301934 | 730362 | 1433976 |
| BtLPMO10B-CD | 1000352 | 478331 | 982513 | |
| BtLPMO10B | 3558191 | 509818 | 1133726 | |
| BtLPMO10A | 4929692 | 4228932 | 7464410 | |
| – | 0 | 0 | 0 | |
Notes:
- -
-
no enzyme was added
H2O2 production of BtLPMO10A, BtLPMO10B and BtLPMO10B-CD
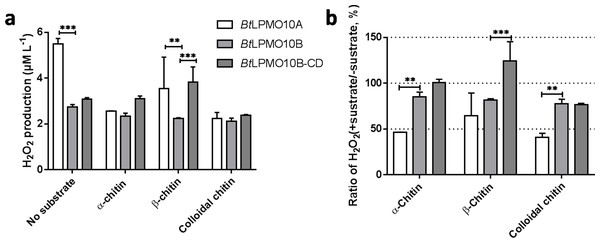
When the substrate was absent, H2O2 produced by BtLPMO10A was much higher than that produced by BtLPMO10B and BtLPMO10B-CD (Figs. 4A and 4B). Accordingly, a much stronger suppression of H2O2 generation in BtLPMO10A was observed as the substrate, especially α-chitin or colloidal chitin, has been provided. Similarly, a mild suppression of H2O2 production by substrate had been observed in the BtLPMO10B, in which all three substrates showed comparable effect. As for the BtLPMO10B-CD, similar inhibition in H2O2 production could be observed when colloidal chitin has been added, while negligible effect on the H2O2 production was recorded when provided with α-chitin or β-chitin.
Figure 4: H2O2 production of BtLPMO10A, BtLPMO10B and BtLPMO10B-CD.
(A) H2O2 production of the enzymes in the absence or presence of diverse substrates. (B) The ratio of H2O2 accumulation in the presence of substrates compared with the absence of substrates.The synergy in chitin degradation
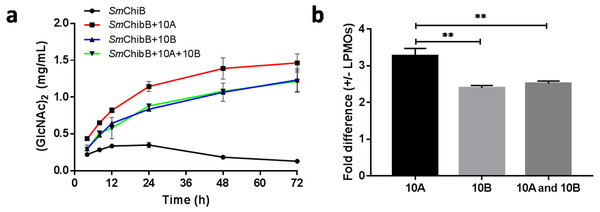
The synergetic effects of BtLPMO10A or BtLPMO10B with SmChiB in α-chitin degradation were performed in the present study (Fig. 5). SmChiB from Serratia marcescens is a model GH18 exo-chitinase which degrades the polymer chains from their non-reducing ends and dominantly produces (GlcNAc)2 (Chen et al., 2020; Van Aalten et al., 2000). The synergy experiment results showed that when only SmChiB was present, the concentration of generated chitobiose reached its plateau (0.336 ± 0.0187 mg/ml) after 12 h of reaction. In contrast, the additional supply of BtLPMO10A or BtLPMO10B can both significantly boost the accumulation of chitobiose, which has reached 1.464 mg ml−1 for BtLPMO10A and 1.232 mg ml−1 for BtLPMO10B, respectively, after 72 h of incubation. Moreover, when both BtLPMO10A and BtLPMO10B were provided, the curve of the concentration of GlcNAc2 over time was similar to that observed when only BtLPMO10B was provided.
Figure 5: Synergetic effects of BtLPMO10A (10A) and BtLPMO10B (10B) to chitinase.
(A) Time course of (GlcNAc)2 production in synergistic or non-synergistic reactions. (B) Fold difference of the production of (GlcNAc)2 at 24 h between the synergistic and non-synergistic reactions.Discussion
The present work was carried out to investigate the biochemical characteristics of BtLPMO10A and BtLPMO10B. It is well known that both BtLPMO10A and BtLPMO10B have a typical AA10 catalytic domain. Sequence and structure analysis indicated that both enzymes have a typical carbohydrate-binding surface and a distinct active site with a phenylalanine rather than tyrosine as the axial residual (Forsberg et al., 2014; Span & Marletta, 2015; Vaaje-Kolstad et al., 2017). The residues in the catalytic domains of both enzymes that may participate in the substrate binding are highly conserved as compared to those found in SmCBP21A (Vaaje-Kolstad et al., 2005) (Fig. 1B). Substrate binding assays showed that BtLPM10A has a similar binding ability to all three chitins tested (α-chitin, β-chitin, and colloidal chitin). In contrast, the multi-modular BtLPMO10B is more inclined to bind to the colloidal chitin. As for the crystalline chitins, BtLPMO10B favors the β-form rather than the α-form, which is contrary to the results reported by Manjeet et al. (2019) in which BtLPMO10B (named BtLPMO10A-FL) prefers to bind the α-form chitin. Furthermore, BtLPMO10B-CD only retained a small portion of the binding abilities of the full-length enzyme toward all three chitins, which are significantly lower than BtLPMO10A. Interestingly, it exhibited comparable binding ability to both α- and β-chitin which is different from the report by Manjeet et al. (2019) that BtLPMO10B-CD showed no binding to α-chitin while retained almost half the binding ability to β-chitin. This suggests that the substrate-binding capacity of BtLPMO10B is mainly contributed by CBM5 which is in accordance to previous reports (Manjeet et al., 2019).
It is worth mention that the binding ability of BtLPMO10B on colloidal chitin is not fully correlated with the activity indicated that certain amount of these binding is non-productive. To verify this possibility, H2O2 concentration in reaction mixtures with different chitins as the substrate were measured, which is based on the knowledge that a productive binding of LPMO to substrate will switch the enzyme from H2O2 production to consumption (Wang, Walton & Rovira, 2019; Zhou et al., 2020). As expected, the concentration of H2O2 showed only mild decrease in all three chitins. Different phenomenon was observed in BtLPMO10A that the H2O2 concentration decreased significantly in the presence of the chitins, indicating its high binding efficiency. Moreover, when using colloidal chitin as the substrate, the H2O2 concentration in the reactions of BtLPMO10B and BtLPMO10B-CD were similar, despite their dramatic difference in binding ability to the substrate. These results indicated that the substrate binding of BtLPMO10B enhanced by CBM5 is not led to enhanced substrate degradation. Therefore, the role of CBM in LPMOs may not just relate with enzyme catalytic activity.
To assess the potential application of BtLPMO10A and BtLPMO10B in chitin preparation, the synergetic effect of the two LPMOs with SmChiB was tested. The results showed that both enzymes can significantly improve the efficiency of the chitinase, similar as observed in other AA10 family LPMOs (Forsberg et al., 2016; Mutahir et al., 2018; Nakagawa et al., 2015; Vaaje-Kolstad et al., 2012). However, BtLPMO10A exhibited much higher efficiency than BtLPMO10B when synergized with SmChiB, which is consistent with the higher activity of BtLPMO10A. Interestingly, when supplied with both BtLPMO10A and BtLPMO10B, the synergetic effect observed was similar to the condition that only supplied with BtLPMO10B, which suggested that the contribution from BtLPMO10A was almost fully suppressed by BtLPMO10B. This may due to the higher binding efficiency of BtLPMO10B on α-chitin which hampered the binding of BtLPMO10A.
Conclusions
In summary, by comparing the structural and biochemical characteristics of BtLPMO10A and BtLPMO10B, we discovered that the two enzymes with highly conserved catalytic domains exhibit different substrate preferences. Further studies indicated that the C-terminal CBM5 domain of BtLPMO10B may be responsible for these diversities implying that the two enzymes may function at different stages in the chitin degradation process. These findings will help us better understand the biological reasons for the LPMO diversity and develop more efficient polysaccharide degrading enzyme cocktails.
Supplemental Information
Quantification of the portions of bound protein as compared to the total protein added
H_2O_2 concentrations
H_2O_2 production of the enzymes in the absence or presence of diverse substrates
(GlcNAc)2 concentrations
Quantification of the concentration of (GlcNAc)2 by HPLC chromatogram.