Integrative taxonomy of Metrichia Ross (Trichoptera: Hydroptilidae: Ochrotrichiinae) microcaddisflies from Brazil: descriptions of twenty new species
- Published
- Accepted
- Received
- Academic Editor
- James Reimer
- Subject Areas
- Biodiversity, Entomology, Taxonomy, Zoology
- Keywords
- Caddisfly, Integrative taxonomy, DNA barcoding, Aquatic insects, Neotropics
- Copyright
- © 2016 Santos et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2016. Integrative taxonomy of Metrichia Ross (Trichoptera: Hydroptilidae: Ochrotrichiinae) microcaddisflies from Brazil: descriptions of twenty new species. PeerJ 4:e2009 https://doi.org/10.7717/peerj.2009
Abstract
Metrichia is assigned to the Ochrotrichiinae, a group of almost exclusively Neotropical microcaddisflies. Metrichia comprises over 100 described species and, despite its diversity, only one species has been described from Brazil so far. In this paper, we provide descriptions for 20 new species from 8 Brazilian states: M. acuminata sp. nov., M. azul sp. nov., M. bonita sp. nov., M. bracui sp. nov., M. caraca sp. nov., M. circuliforme sp. nov., M. curta sp. nov., M. farofa sp. nov., M. forceps sp. nov., M. formosinha sp. nov., M. goiana sp. nov., M. itabaiana sp. nov., M. longissima sp. nov., M. peluda sp. nov., M. rafaeli sp. nov., M. simples sp. nov., M. talhada sp. nov., M. tere sp. nov., M. ubajara sp. nov., and M. vulgaris sp. nov. DNA barcode sequences (577 bp of the mitochondrial gene COI) were generated for 13 of the new species and two previously known species of Metrichia resulting in 64 sequences. In addition, COI sequences were obtained for other genera of Ochrotrichiinae (Angrisanoia, Nothotrichia, Ochrotrichia, Ragatrichia, and Rhyacopsyche). DNA sequences and morphological data were integrated to evaluate species delimitations. K2P pairwise distances were calculated to generate a neighbor-joining tree. COI sequences also were submitted to ABGD and GMYC methods to assess ‘potential species’ delimitation. Analyses showed a conspicuous barcoding gap among Metrichia sequences (highest intraspecific divergence: 4.8%; lowest interspecific divergence: 12.6%). Molecular analyses also allowed the association of larvae and adults of Metrichia bonita sp. nov. from Mato Grosso do Sul, representing the first record of microcaddisfly larvae occurring in calcareous tufa (or travertine). ABGD results agreed with the morphological delimitation of Metrichia species, while GMYC estimated a slightly higher number of species, suggesting the division of two morphological species, each one into two potential species. Because this could be due to unbalanced sampling and the lack of morphological diagnostic characters, we have maintained these two species as undivided.
Introduction
The microcaddisfly genus Metrichia Ross, 1938 is included in the subfamily Ochrotrichiinae, which also includes Ochrotrichia Mosely, 1934, Angrisanoia Özdikmen, 2008, Nothotrichia Flint, 1967, Rhyacopsyche Müller, 1879, and the recently erected Ragatrichia Oláh & Johanson, 2011, all of them exclusively from New World. Based on adult morphology, Harris & Armitage (1997) and Oláh & Johanson (2011) also suggested three other genera to be included in this subfamily: Dibusa Ross, 1939, Caledonotrichia Sykora, 1967, and Maydenoptila Neboiss, 1977, from the USA, New Caledonia, and Australia, respectively. However, because diversification of main lineages of hydroptilids has not been deeply studied, the placement of these genera remains dubious. As noted by Wells, Johanson & Mary-Sasal (2013), relationships of these microcaddisflies need to be studied based on rigorous analyses including molecular data.
Currently, Metrichia includes 107 species, found from the USA to South America, with highest known diversity in Central America (Flint Jr, 1972; Marshall, 1979). Metrichia was considered as a subgenus of Ochrotrichia due to similarities of adult morphology and almost indistinguishable larvae (Flint Jr, 1968). This subgeneric status was followed by Marshall (1979), who also established the New World tribe that is now recognized as subfamily Ochrotrichiinae. Wiggins (1996) provided additional information on larval morphology of Metrichia and Ochrotrichia, reestablishing both as independent genera.
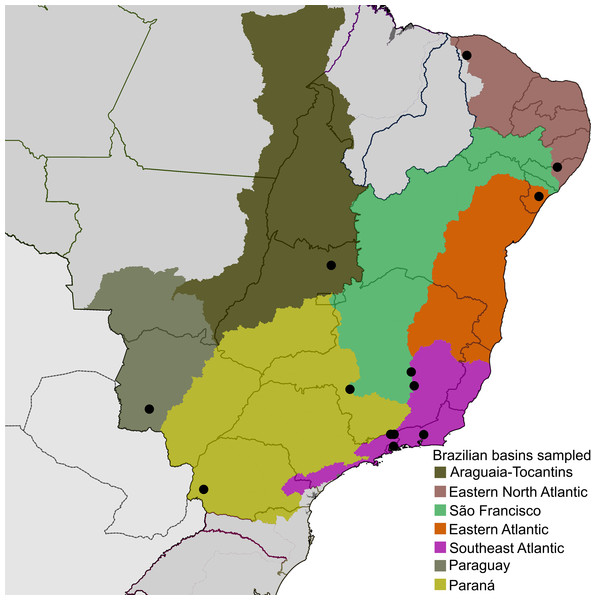
Diversity of Neotropical microcaddisflies is poorly known and usually several undescribed species are found in collections or amongst recently collected material when examined by experts, even in localities previously studied by trichopterologists. This likely occurs because Hydroptilidae are very small and have complex male genitalia, making them difficult to observe by lower magnification microscopes and to understand homologies among some structures. Only one species of Metrichia has been described from Brazil so far, M. pernambucana Souza & Santos, 2013, but larvae have been commonly identified from several localities (e.g., Pes, Hamada & Nessimian, 2005; Spies & Froehlich, 2009; Spies, Froehlich & Kotzian, 2006). It is not surprising that material studied herein recently collected from different river basins in Brazil (Fig. 1, Supplemental Information 1 and 2) revealed so many undescribed species.
Figure 1: Localities (●), distributed on seven of the large river basins of Brazil, where Metrichia specimens studied herein were collected.
Most Metrichia species exhibit a more restricted distribution, in other words, each species has been recorded only from type-localities or relatively few close localities. Herein we describe one species with a wide distribution in Brazil, occurring in three very distinctive biomes: Atlantic Forest (Southeastern Brazil), Caatinga (Northeastern Brazil), and Cerrado (Centralwestern Brazil). Although such wide distributions are not common in Metrichia, similar examples are known for other Neotropical microcaddisflies, as Oxyethira tica Holzenthal & Harris, 1992, recorded from Mexico, Central, and South America (Flint Jr, Holzenthal & Harris, 1999).
Although molecular tools have become common in taxonomic studies to help in species delimitation, their use is still rare with Neotropical caddisflies. Using sequences of the mitochondrial cytochrome oxidase I gene (COI), the standard DNA barcode region for animals (Hebert et al., 2003), Pauls et al. (2010) were able to corroborate two new species of Smicridea (Smicridea) McLachlan, 1871 from Chile, defined with morphological characters. In most studies with caddisflies, divergence in COI sequences has shown clear differences between intraspecific and interspecific variation, the so-called ‘barcoding gap’ (Zhou, Kjer & Morse, 2007; Pauls et al., 2010; Ruiter, Boyle & Zhou, 2013). Some species delimitation approaches rely solely in distinguishing intra- and interspecific divergence, such as the Automatic Barcoding Gap Discovery (ABGD) (Puillandre et al., 2012). More sophisticated methods invoke coalescence and speciation models, such as the General Mixed Yule Coalescent (GMYC), and are considered more robust for identifying lineages when intra- and interspecific divergences overlap (Pons et al., 2006). Integrating independent data (e.g., morphology and DNA sequences) and using different approaches are particularly interesting for the taxonomy of diverse and complex groups, such as microcaddisflies. In this work, we applied both ABGD and GMYC methodologies to evaluate our initial morphological identification.
Besides its use for species delimitation, DNA taxonomy has a valuable role in making associations between immature and adult stages of caddisflies (Graf, Lubini & Pauls, 2005; Waringer et al., 2007; Zhou, Kjer & Morse, 2007; Ruiter, Boyle & Zhou, 2013). Traditional techniques to associate larvae and adults are more difficult because they involve rearing larvae in the laboratory (not an easy task for many caddisfly groups) or luck in finding pharate adults in field. Indirect association of stages, for example, by collecting adults and larvae at the same locality, can result in misidentification, because different species of the same genus frequently co-occur.
Larvae of Metrichia have been associated for only two species: M. nigritta (Banks, 1907) described by Edwards & Arnold (1961) and illustrated by Wiggins (1996), and M. juana (Flint Jr, 1964) by Flint Jr (1964) in the original description. In addition, Botosaneanu & Flint Jr (1982) described a larva of Metrichia and its case from Venezuela and a pupal case from Ecuador; and Pes, Hamada & Nessimian (2005) illustrated larvae from Brazil and three different types of cases. In both works, specific names were not provided as authors did not have respective adults. Metrichia larvae build a typical oval purse-like case, made of silk, usually covered with algae filaments (Wiggins, 1996; Pes, Hamada & Nessimian, 2005), and sometimes also having sand grains (Botosaneanu & Flint Jr, 1982). Cases of some larvae that have not yet been associated with adults show a pair of dorsal “chimneys,” an uncommon feature also described and illustrated by Müller (1879) and Müller (1880) for Dicaminus ladislavii Müller, 1879, from Santa Catarina, Brazil. Based on larval cases from Central and South America, Botosaneanu & Flint Jr (1982) pointed that Dicaminus is possibly synonymous with Metrichia, but this question remains open, since there are no male specimens from Müller’s work.
Almost nothing is known about the biology of Metrichia larvae. According to Wiggins (1996), larvae of M. nigritta were collected in association with filamentous algae on rock surfaces. In Brazil, Metrichia occurs in fast flowing streams, usually with associated algae. Herein, we report for the first time the occurrence of Metrichia in calcareous tufa. Calcareous tufa or travertine is a terrestrial sedimentary rock, predominantly composed of carbonate minerals, calcite, and aragonite (Drysdale, 1998). Travertines are formed by rapid precipitation of these minerals, producing large alterations on river morphology (Drysdale & Gale, 1997). Although the importance of microbes on travertine formation is recognized by creating surfaces for crystal nucleation, our knowledge of the importance of macroinvertebrates in this process is still poor (Drysdale, 1998; Drysdale, 1999; Paprocki, Holzenthal & Cressa, 2003). Studies by Drysdale (1998) and Drysdale (1999) pointed out that aquatic insects play an important role in travertine biogenesis in Australian springs, especially Cheumatopsyche Wallengreen, 1891 (Hydropsychidae) larvae. Paprocki, Holzenthal & Cressa (2003) also found another Hydropsychidae (Smicridea) to be an important organism in modifying travertine morphology in Venezuela.
Material and Methods
Morphological study
Specimens were collected manually (larvae or diurnal active adults) or using Malaise or light traps, and then fixed in 96% ethanol. Collecting permits in Brazil were issued by Instituto Chico Mendes de Conservação da Biodiversidade (ICMBio) (SISBIO 43047 and 14591). To observe genital structures, abdomen of males were removed and cleared in a heated solution of 10% KOH for 20 min. Then, abdomens were mounted in temporary slides, which were used to draw pencil sketches with compound microscope equipped with camera lucida. Vector graphics were traced in Adobe Illustrator CS6 (Adobe Systems Inc.) using pencil sketches as templates. Descriptions provided here were made with DELTA software (Description Language for Taxonomy) (Dallwitz, Paine & Zurcher, 1999). Terminology used throughout this paper follows that provided by Marshall (1979) and Bueno-Soria & Holzenthal (2003). Types for newly described species are deposited at Coleção Entomológica Prof. José Alfredo Pinheiro Dutra, Departamento de Zoologia, Universidade Federal do Rio de Janeiro, Rio de Janeiro (DZRJ); Museu Nacional, Universidade Federal do Rio de Janeiro, Rio de Janeiro (MNRJ); Instituto Nacional de Pesquisas da Amazônia, Manaus (INPA); Coleção Zoológica do Maranhão (CZMA), Caxias; and Museu de Zoologia da Universidade Federal da Bahia, Salvador (MZUFBA).
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:D8D4049E-494B-4A30-92AC-F8F42D2B54B9. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
DNA extraction, PCR, and sequencing
Genomic DNA was extracted from head and thorax (or from the entire body) of fresh material using the DNeasy Blood and Tissue Kit (QIAGEN, Hilden, Germany), without tissue maceration. After extraction, specimens were returned to ethanol and deposited in DZRJ collection as a DNA voucher. COI fragments were amplified using pair of primers: HCO-2198 (5′-TAAACTTCAGGGTGACCAAAAAATCA-3′) in combination with LCO-1490 (5′-GGTCAACAAATCATAAAGATATTGG-3′) (Folmer et al., 1994) or C1-J-1718 (5′-GGAGGATTTGGAAATTGATTAGTTCC-3′) (Simon et al., 1994). Polymerase chain reaction (PCR) conditions were as follows: initial denaturation at 94 °C for 3 min; 35 cycles of denaturation at 94 °C for 1 min, annealing at 50 °C for 1 min, and extension at 72 °C for 2 min; and final extension at 72 °C for 7 min. PCR products were sent to Macrogen Inc., Seoul, for purification and sequencing reactions.
COI sequences of 64 specimens of 15 species of Metrichia were obtained. Additional sequences were obtained for specimens of Angrisanoia, Nothotrichia, Ochrotrichia, and Rhyacopsyche (Table 1), included as outgroup in different analyses, as described below.
| Species | Voucher code and life stage | Collection site | GenBank accession number |
|---|---|---|---|
| Angrisanoia cebollati (Angrisano, 1995) | ENT 2199 ♂ | Brazil: Goiás: Alto Paraíso de Goiás | – |
| Betrichia bispinosa Flint, 1974 | ENT 2337 ♂ | Brazil: Amapá | KU094961b |
| Nothotrichia cautinensis Flint, 1983 | – | – | KC559534a |
| Nothotrichia tupi Holzenthal & Harris, 1992 | ENT 2460 ♂ | Brazil: Minas Gerais: Catas Altas | KU743400 |
| Ochrotrichia caatinga Souza, Santos & Takiya, 2014 | ENT 2472 ♂ | Brazil: Ceará: Ubajara | KU743401 |
| Ochrotrichia patulosa (Wasmund & Holzenthal, 2007) | ENT 2473 ♂ | Brazil: Ceará: Ubajara | KU743402 |
| Ochrotrichia sp. CR1 | ENT 2279 ♂ | Costa Rica: Puntarenas | KU094950b |
| Oxyethira tica Holzenthal & Harris, 1992 | ENT 0057 ♂ | Brazil: Pará: Carajás | KU094940b |
| Ragatrichia sp. BR1 | ENT 2338 ♂ | Brazil: Amapá | KU743403 |
| Rhyacopsyche dikrosa Wasmund & Holzenthal, 2007 | ENT 0122 ♂ | Brazil: Rio de Janeiro: Teresópolis | KU094952b |
| Rhyacopsyche torulosa Flint, 1971 | ENT 2277 ♂ | Costa Rica: Puntarenas | KU743404 |
| Metrichia acuminata sp. nov. | ENT 2192 ♂ | Brazil: Alagoas: Quebrangulo | KU743406 |
| ENT 2282 ♂ | KU743427 | ||
| ENT 2284-5 ♂ | KU743428– KU743429 | ||
| ENT 2779 ♂ | KU743452 | ||
| Metrichia amplitudinis Bueno-Soria & Holzenthal, 2003 | ENT 2278 ♂ | Costa Rica: Puntarenas | KU743425 |
| Metrichia bonita sp. nov. | ENT 2200-4 larvae | Brazil: Mato Grosso do Sul: Bonito | KU743409, KU743410, KU743411, KU743412, KU743413 |
| Metrichia bonita sp. nov. | ENT 2208-10 ♂ | Brazil: Mato Grosso do Sul: Bonito | KU743417, KU743418, KU743419 |
| Metrichia bracui sp. nov. | ENT 2508-11 ♂ | Brazil: Rio de Janeiro: Itatiaia | KU743444, KU743445, KU743446, KU743447 |
| Metrichia caraca sp. nov. | ENT 2195 ♂ | Brazil: Minas Gerais: Catas Altas | KU743408 |
| ENT 2280 ♂ | KU743426 | ||
| ENT 2461-5 ♂ | KU743434, KU743435, KU743436, KU743437, KU743438 | ||
| Metrichia caraca sp. nov. | ENT 2292 ♂ | Brazil: Minas Gerais: São Roque de Minas | KU743432 |
| Metrichia circuliforme sp. nov. | ENT 2835-7 ♂ | Brazil: Rio de Janeiro: Itatiaia | KU743455, KU743456, KU743457 |
| ENT 2839-40 ♂ | KU743459– KU743460 | ||
| ENT 2843-4 ♂ | KU743462– KU743463 | ||
| Metrichia curta sp. nov. | ENT 2838 ♂ | Brazil: Rio de Janeiro: Itatiaia | KU743458 |
| ENT 2846-8 ♂ | KU743464, KU743465, KU743466 | ||
| Metrichia formosinha sp. nov. | ENT 2205-7 ♂ | Brazil: Mato Grosso do Sul: Bonito | KU743414, KU743415, KU743416 |
| Metrichia itabaiana sp. nov. | ENT 2190 ♂ | Brazil: Sergipe: Areia Branca | KU743405 |
| Metrichia itabaiana sp. nov. | ENT 2220-1 ♂ | Brazil: Goiás: Alto Paraíso de Goiás | KU743424 |
| Metrichia juana (Flint, 1964) | ENT 2850-1 ♂ | Puerto Rico | KU743467– KU743468 |
| Metrichia longissima sp. nov. | ENT 2330 ♂ | Brazil: Rio de Janeiro: Teresópolis | KU743433 |
| Metrichia longissima sp. nov. | ENT 2841 ♂ | Brazil: Rio de Janeiro: Itatiaia | KU743461 |
| Metrichia rafaeli sp. nov. | ENT 2288-9 ♂ | Brazil: Ceará: Ubajara | KU743430– KU743431 |
| Metrichia talhada sp. nov. | ENT 2193 ♂ | Brazil: Alagoas: Quebrangulo | KU743407 |
| ENT 2214 ♂ | KU743420, KU743421, KU743422 | ||
| ENT 2216-7 ♂ | KU743451 | ||
| ENT 2776 ♂ | |||
| Metrichia tere sp. nov. | ENT 2773-5 ♂ | Brazil: Rio de Janeiro: Teresópolis | KU743448, KU743449, KU743450 |
| Metrichia vulgaris sp. nov. | ENT 2218 ♂ | Brazil: Goiás: Alto Paraíso de Goiás | KU743423 |
| Metrichia vulgaris sp. nov. | ENT 2466-70 ♂ | Brazil: Minas Gerais: Catas Altas | KU743439, KU743440, KU743441, KU743442, KU743443 |
| Metrichia vulgaris sp. nov. | ENT 2833-4 ♂ | Brazil: Rio de Janeiro: Itatiaia | KU743453– KU743454 |
Notes:
Sequences obtained from GenBank.
Sequence editing, alignment, and analyses
Forward and reverse sequences of each sample were assembled and manually edited in Sequencher 4.1 (Gene Codes, Ann Arbor, Michigan, USA). Sequences were verified with the Blast tool in GenBank to check for contamination. Subsequently, COI sequences were aligned with ClustalW implemented in MEGA 6 (Tamura et al., 2013) and translated into amino-acid sequences to check for stop codons. The final alignment resulted in a matrix with 577 bp (Supplemental Information 3).
COI sequences were used to explore putative species limits with four different methodologies: (1) lineages recovered in neighbor-joining tree; (2) lineages recovered with Bayesian Inference; (3) ABGD; and (4) GMYC. The neighbor-joining tree was calculated in MEGA 6 using Kimura 2-Parameter (K2P) distances (Kimura, 1980), with partial deletion of missing information. Although the use of K2P distances in DNA barcoding is debated (Srivathsan & Meier, 2012), to allow comparison with previous works we also used this evolutionary model because it is frequently used in studies of species delimitation based on COI sequences. Branch support of neighbor-joining tree was assessed with 1,000 pseudoreplicates of non-parametric bootstrap (Felsenstein, 1985).
BI analysis was conducted with MrBayes v. 3.2.2 (Ronquist et al., 2012) with four independent runs, each one with four MCMC chains running for 50,000,000 generations, with sample frequency of 5,000. Convergence of sampled parameters was checked in Tracer v. 1.5 (Rambaut & Drummond, 2007) and the first 10% of sampled trees and parameters discarded as burnin. GTR + I + G was the best fit model selected by Akaike Information Criterion (AIC) with jModeltest v. 0.1.1 (Posada, 2008) and it was applied in BI analysis in MrBayes. Branch support was assessed by posterior probability (PP), presented on a 50% majority consensus tree.
ABGD analysis was run using the on-line version available in http://wwwabi.snv.jussieu.fr/public/abgd/, where the COI alignment was uploaded. The analysis was conducted with the following settings: Pmin = 0.001; Pmax = 0.1; steps = 20; relative gap width = 1.0, also based on K2P model. This method statistically infers the DNA barcode gap in a single locus alignment, partitioning the data based on this gap in putative species (Puillandre et al., 2012).
The GMYC analysis (Pons et al., 2006; Fujisawa & Barraclough, 2013) was performed in R (R Development Core Team, 2010) using the SPLITS package (Ezard, Fujisawa & Barraclough, 2009) with single-threshold method. Basically, the method estimates branching patterns on an ultrametric tree, identifying the most likely transition point from coalescent to speciation branching. The ultrametric tree used here was obtained with BEAST v. 1.8 (Drummond et al., 2012) under a relaxed uncorrelated molecular clock (Drummond et al., 2006). The node including Ochrotrichia species was calibrated based on fossil evidence with a lognormal distribution offset at 20 mya and log(mean) = 2.8 to represent the possible range of 20–140 mya (Wells & Wichard, 1989); and the divergence of Ochrotrichiinae was calibrated based on Malm, Johanson & Wahlberg (2013) with a normal distribution with mean 82.17 ± 12 mya. The BEAST analysis ran for 200,000,000 generations, sampled every 10,000 generations. Convergence was verified with Tracer and a maximum credibility tree was written using TreeAnotator, discarding the first 10% as burnin.
Results
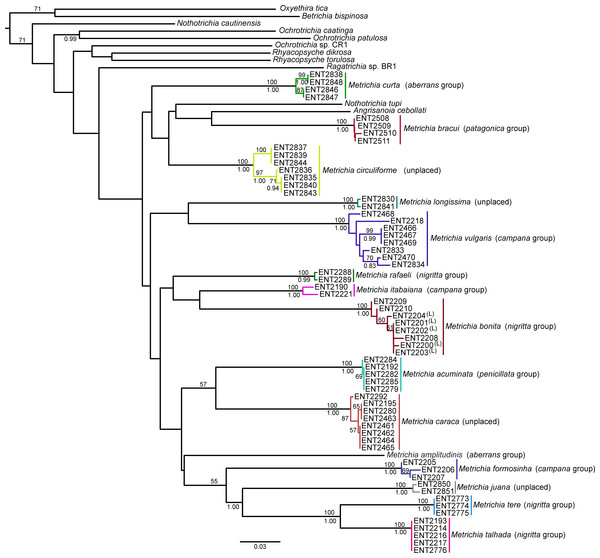
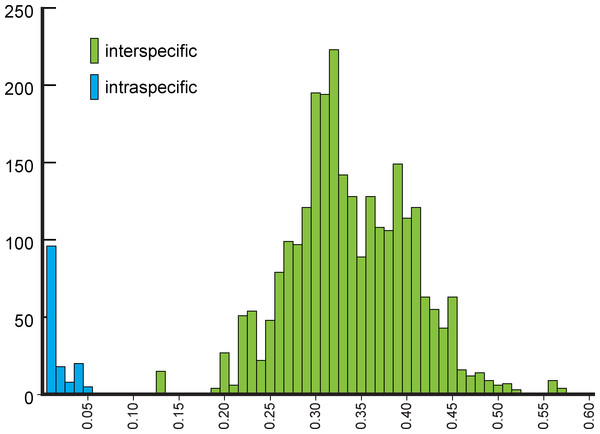
NJ (Fig. 2) and BI (Supplemental Information 4) trees corroborated morphological identification, with all 14 species of Metrichia with more than a single specimen recovered as monophyletic lineages with 100% bootstrap support. ABGD also returned the same species as they were previously delimited based on morphological features. A robust ‘barcoding gap’ was found among Metrichia species (Fig. 3 and Table 2). The maximum intraspecific divergence was observed within Metrichia vulgaris sp. nov. (0.048). The minimum interspecific divergence was found between specimens of Metrichia talhada sp. nov. and Metrichia tere sp. nov. (0.126).
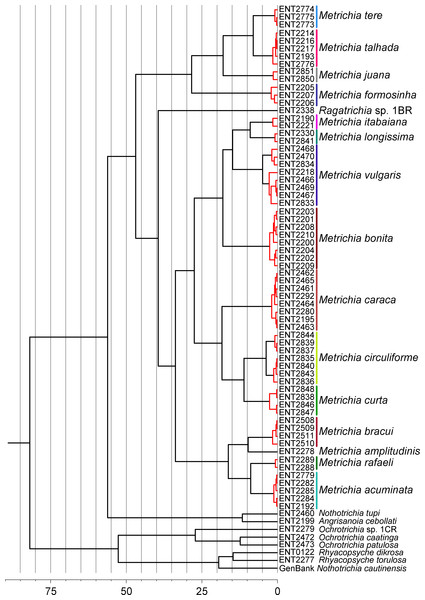
GMYC analysis estimated a slightly higher number of putative species, with Metrichia circuliforme sp. nov. and Metrichia vulgaris sp. nov. being each further divided into two species (Fig. 4). Regarding all other species, GMYC results were congruent with other methods and with morphology.
Figure 2: Neighbor-joining tree of COI sequences of Metrichia species based on K2P distances.
Numbers above and below branches are, respectively, NJ bootstrap support and posterior probabilities from BI analysis. Details of specimens are in Tables 1 and 2; K2P distances matrix is in Supplemental Information 5.Figure 3: Histogram of the number of pairwise comparisons of intra- (blue) and interspecific (green) K2P divergences among 15 Metrichia species with COI sequences sampled.
| Species | Number of sequences | Max. intra. distance | Min. inter. distance |
|---|---|---|---|
| M. acuminata sp. nov. | 5 | 0.000 | 0.217 |
| M.amplitudinisBueno-Soria & Holzenthal, 2003 | 1 | – | 0.220 |
| M.bonita sp. nov. | 8 | 0.015 | 0.210 |
| M.bracui sp. nov. | 4 | 0.004 | 0.214 |
| M. caraca sp. nov. | 8 | 0.011 | 0.217 |
| M.circuliforme sp. nov. | 7 | 0.035 | 0.184 |
| M.curta sp. nov. | 4 | 0.015 | 0.184 |
| M.formosinha sp. nov. | 3 | 0.008 | 0.249 |
| M.itabaiana sp. nov. | 2 | 0.019 | 0.194 |
| M.juana (Flint, 1964) | 2 | 0.007 | 0.243 |
| M.longissima sp. nov. | 2 | 0.004 | 0.215 |
| M.rafaeli sp. nov. | 2 | 0.004 | 0.194 |
| M.talhada sp. nov. | 5 | 0.000 | 0.126 |
| M.tere sp. nov. | 3 | 0.000 | 0.126 |
| M.vulgaris sp. nov. | 8 | 0.048 | 0.246 |
Figure 4: Maximum credibility time tree from the BEAST analysis based on COI sequences of Metrichia and other Ochrotrichiinae.
Red branches represent species estimated by GMYC using single threshold in SPLITS. Timescale in millions of years.In all analyses performed using DNA barcode, Metrichia larvae collected in calcareous tufa were consistently associated with adult males of Metrichia bonita sp. nov. Therefore, in the following section, we describe these larvae within that species.
Species Descriptions
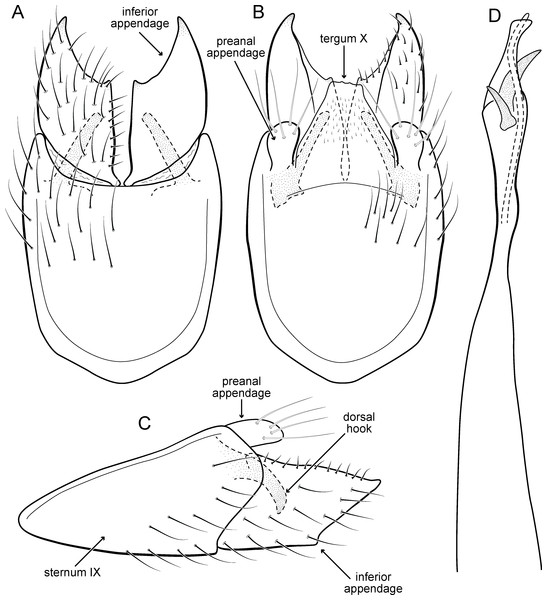
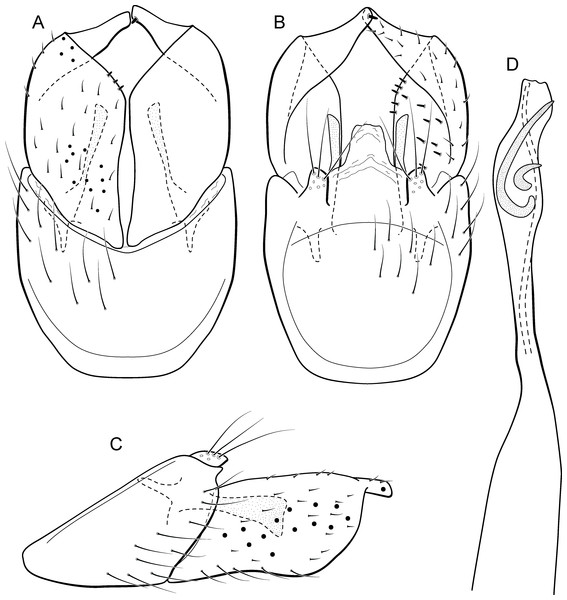
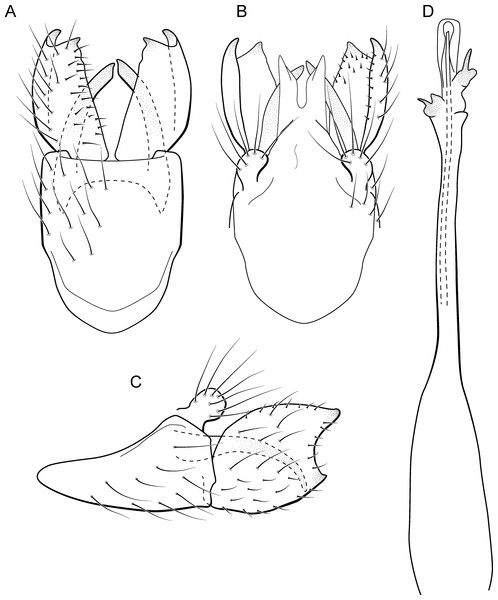
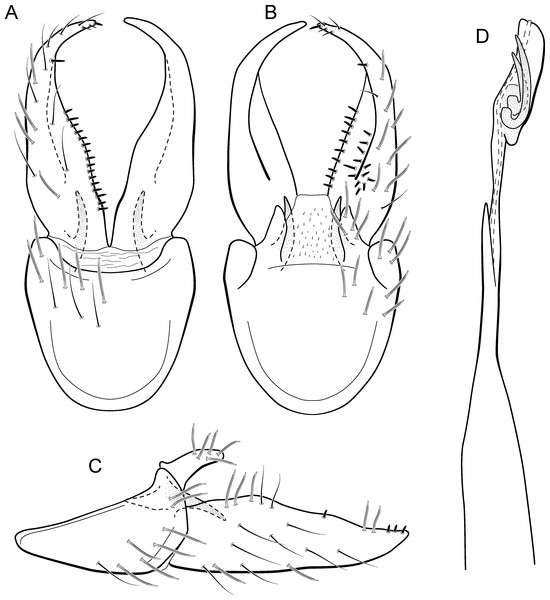
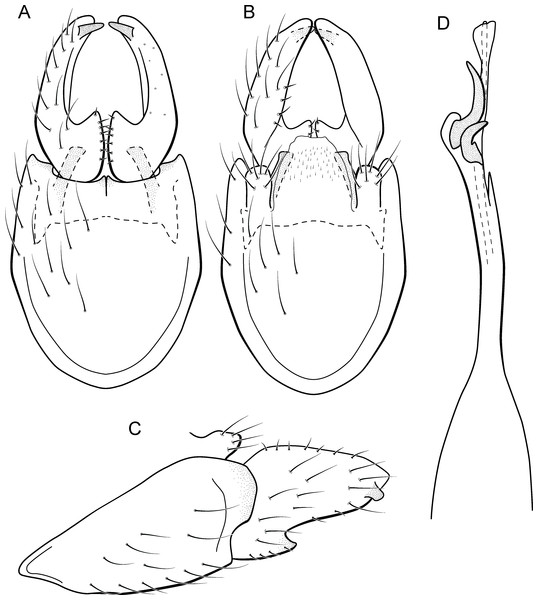
Metrichia acuminata sp. nov.
urn:lsid:zoobank.org:act:01493211-CD39-4995-B2D7-8F3B44A0970E
Figure 5: Metrichia acuminata sp. nov., male genitalia.
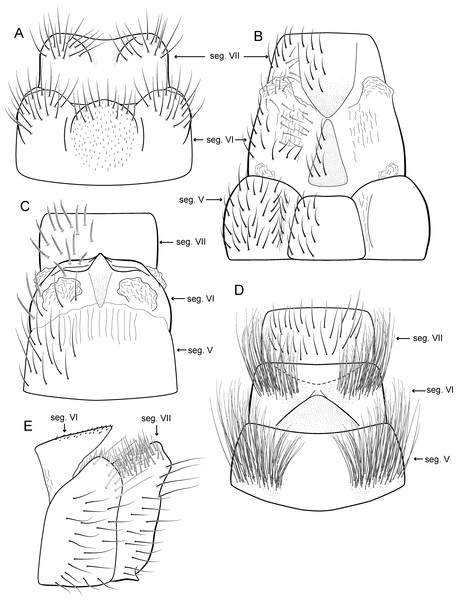
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 2.1–2.5 mm (n = 5). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Antenna simple, 18-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment IV with pair of internal pouches in posterior area; segment V with pair of internal pouches; segment VI with tergum as a sclerotized rounded plate, surrounded by long setae (Fig. 26A); segment VII bearing a brush of very long setae dorsolateraly (Fig. 26A). Ventromesal process on segment VII present. Segment VIII shorter ventrally than dorsally and bearing a brush of long setae dorsally. Male genitalia. Segment IX reduced dorsally; sternum subrectangular, with anterior margin rounded (Fig. 5A); in lateral view narrower anteriorly than posteriorly (Fig. 5C). Inferior appendage covered by long setae; subtrapezoidal in ventral view (Fig. 5A); in lateral view, subtrapezoidal, apex with acute corners (Fig. 5C). Dorsal hook short, almost half length of inferior appendage; in lateral view, downturned (Fig. 5C). Preanal appendage rounded in lateral view and bearing very long setae (Fig. 5C). Subgenital plate apparently absent. Tergum X membranous and truncate (Fig. 5B). Phallus tubular, elongate and slender, slightly constricted mesally, with two curved subapical spines, one short and another long; apex truncate and sclerotized; ejaculatory duct sclerotized, sinuous, and protruding apically (Fig. 5D).
Holotype. BRAZIL: Ceará: Ubajara, Parque Nacional de Ubajara, Cachoeira do Gameleira, , el. 880 m, 23.iv.2012, DM Takiya & JA Rafael cols., light trap, male (CZMA).
Paratypes. Same data as holotype, except, Rio das Minas, , el. 524 m, 13–17.ix.2012, JA Rafael et al., Malaise trap, 2 males (INPA); same data, except 14–16.ii.2013, DM Takiya, JA Rafael, RR Cavichioli & APM Santos, Malaise trap, 2 males (DZRJ). Alagoas: Quebrangulo, Reserva Biológica de Pedra Talhada, Rio Caranguejo, , el. 550 m, 19–28.vi.2014, APM Santos, DM Takiya, WRM Souza, Malaise trap, 2 males (MNRJ), 3 males (MZUFBA), 13 males (DZRJ).
Etymology. The species is named in allusion to the pointed apices of inferior appendages (from Latin, “acumin-” = “pointed”).
Remarks. This new species belongs to the penicillata group based on: (1) internal pouches between abdominal segments IV and V; (2) setal brushes on segments V, VI, and VII; and (3) phallus with two subapical spines. The male genitalia and complex abdominal modifications resemble M. penicillata (Flint Jr, 1972) and M. trigonella (Flint Jr, 1972). These three species have inferior appendages with acute apices in lateral view; phallus with two subapical spines; and abdominal terga with brushes of very long and stout setae. The new species can be distinguished by inferior appendages more trapezoidal in lateral view, with acute corners posteriorly and dorsal hook only slightly downturned in lateral view; and phallus with one larger and stouter subapical spine. Although the male genitalia of this new species superficially resemble that of M. bonita sp. nov., M. acuminata sp. nov. is readily recognized by setose lobes on abdominal segments V and VI.
We were not able to obtain COI sequences for individuals from Ceará State, so the five sequences analyzed belong to specimens from Alagoas State, which shared the same haplotype. Metrichia acuminata sp. nov. was recovered as closely related to M. caraca sp. nov. (Fig. 2), but in both Bayesian approaches these two species were not recovered as sister taxa (Fig. 4, Supplemental Information 1).
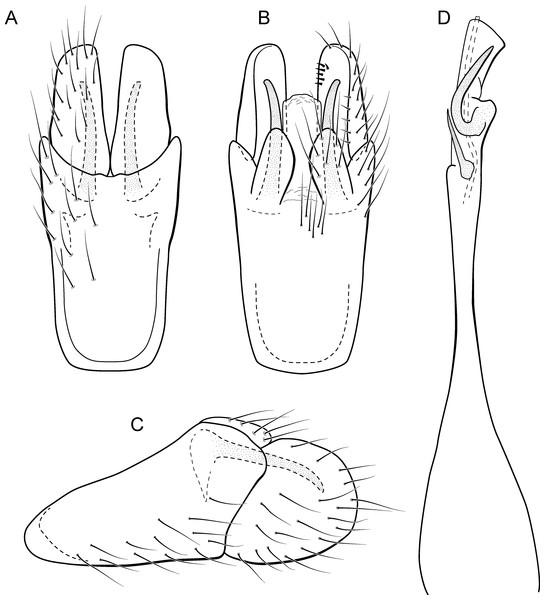
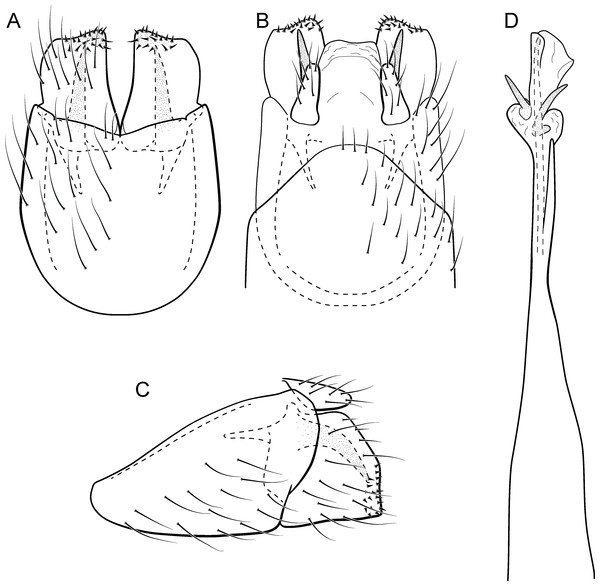
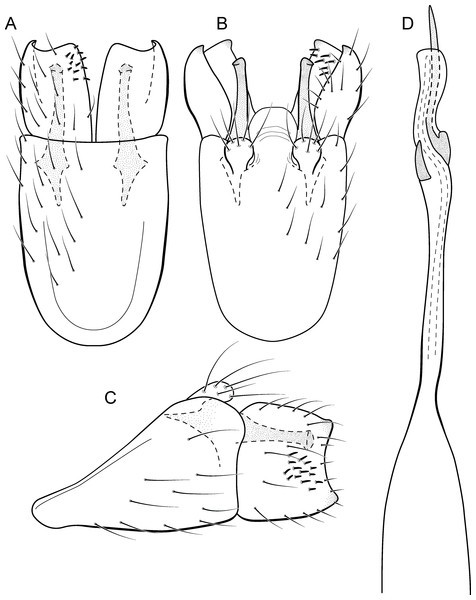
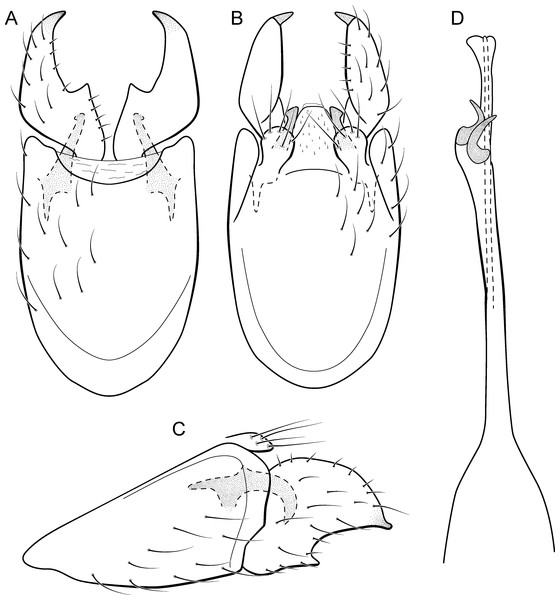
Metrichia azul sp. nov.
urn:lsid:zoobank.org:act:E38ACAE9-61DC-4B61-A20D-1879520E1DD3
(Fig. 6)
Figure 6: Metrichia azul sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 2.0–2.1 mm (n = 4). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Maxillary palpus 5-articulated, article IV broad and darkened; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment V with pair of internal pouches; segment VI with pair of internal pouches and pair of lateral external sacs with specialized setae. Ventromesal process on segment VII present. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subrectangular (Fig. 6A); in lateral view narrower anteriorly than posteriorly (Fig. 6C). Inferior appendage covered by long setae and with scale-like setae; subrectangular in ventral view (Fig. 6A); in lateral view, rounded, apex rounded (Fig. 6C). Dorsal hook long, more than half length of inferior appendage; in lateral view, slightly downturned (Fig. 6C). Preanal appendage elongate, but shorter than inferior appendage, and bearing very long setae (Fig. 6B). Subgenital plate apparently absent. Tergum X membranous and subrectangular (Fig. 6B). Phallus tubular, elongate and slender, slightly constricted mesally, with two long, curved, subapical spines; apex rounded and sclerotized; ejaculatory duct sclerotized, sinuous, and protruding apically (Fig. 6D).
Holotype. BRAZIL: Paraná: Céu Azul, Parque Nacional do Iguaçu, Rio Azul, , el. 510 m, 6–8 ix.2012, APM Santos, DM Takiya, ALH Oliveira, GA Jardim & BHL Sampaio cols., Malaise trap, male (DZRJ).
Paratypes. Same data as holotype, 2 males (DZRJ), 1 male (MNRJ).
Etymology. The specific name refers to the type locality, Rio Azul in the municipality of Céu Azul.
Remarks. This new species is another member of the penicillata group based on internal pouches between segment V and VI and the long subapical spines of the phallus. The new species shares similarities of the male genitalia with M. biungulata (Flint Jr, 1972) and M. decora Bueno-Soria & Holzenthal, 2003 particularly the rounded aspect of inferior appendages, but can be easily distinguished from those species by the absence of tooth-like processes on inferior appendages; more elongate preanal appendages; and dorsal hook only slightly downturned in lateral view.
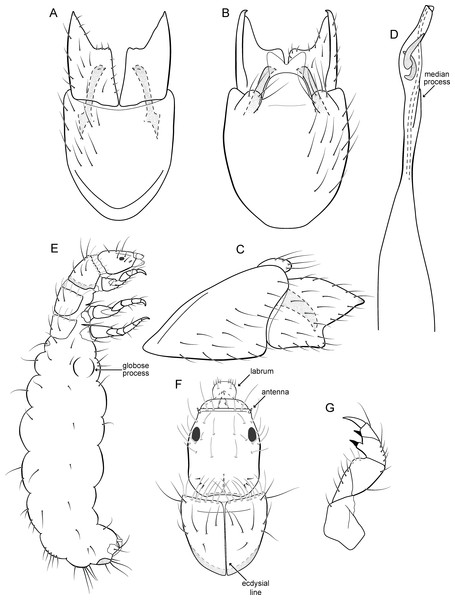
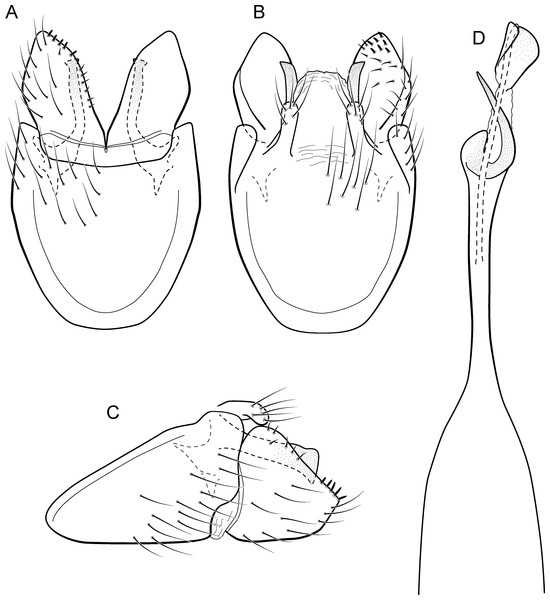
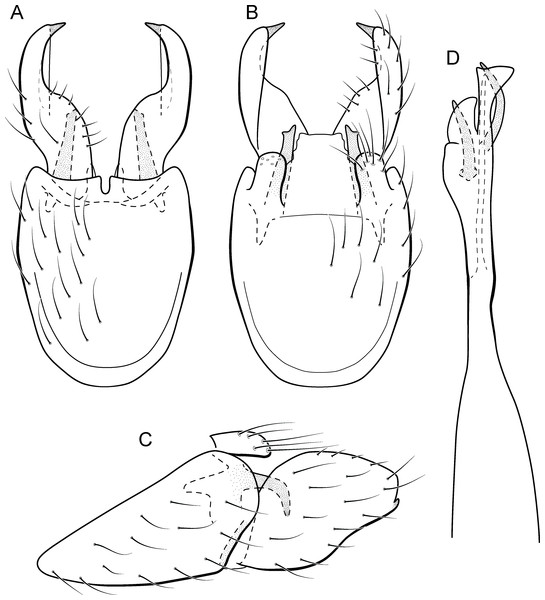
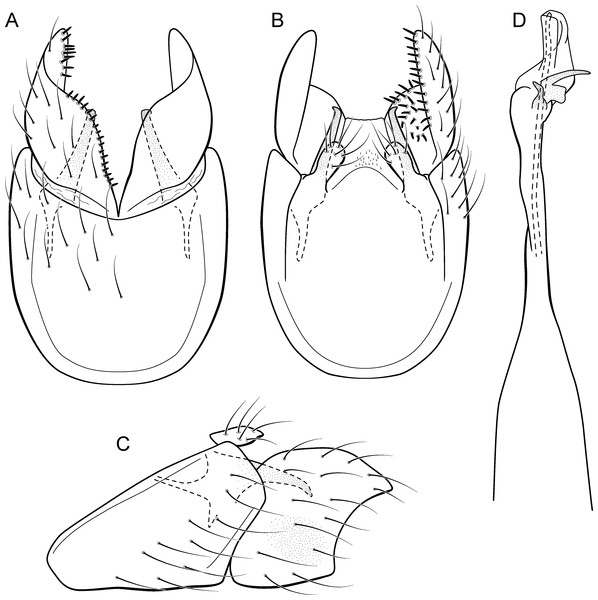
Metrichia bonita sp. nov.
urn:lsid:zoobank.org:act:622BCD51-CC39-4D1F-BDCB-84A7DFF4F071
Figure 7: Metrichia bonita sp. nov.
(A) male genitalia, ventral view; (B) male genitalia, dorsal view; (C) male genitalia, lateral view; (D) phallus, dorsal view; (E) larva, habitus, lateral view; (F) larva, head and pronotum, dorsal view; (G) larva, foreleg, ventral view.Figure 8: Metrichia bonita sp. nov., larva.
(A) type locality, Rio Formosinho, Bonito municipality, Mato Grosso do Sul, Brazil; (B) larvae on calcareous substrate; (C) larva and its calcareous case.Adult male. Length 2.3–2.5 mm (n = 4). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Antenna simple, 21-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment VI with dorsal pouches covered with setae directed inward. Ventromesal process on segment VII absent. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subrectangular, with anterior margin rounded (Fig. 7A); in lateral view narrower anteriorly than posteriorly (Fig. 7C). Inferior appendage covered by long setae, subtrapezoidal in ventral view (Fig. 7A); in lateral view, with an acute projection, apex with acute corners (Fig. 7C). Dorsal hook short, almost half length of inferior appendage; in lateral view, slightly downturned (Fig. 7C). Preanal appendage short, rounded and bearing very long setae (Fig. 7B). Subgenital plate apparently absent. Tergum X membranous and with a shallow U-shaped incision (Fig. 7B). Phallus tubular, elongate and slender, slightly constricted mesally, with a median process; with two curved subapical spines, one short and another long; apex truncate and sclerotized; ejaculatory duct sclerotized and not protruding apically (Fig. 7D).
Larva (5th instar). Length 1.5–1.9 mm (n = 10). Head dark brown, unpigmented around stemmata (Fig. 8C); slightly longer than broad; frontoclypeal and coronal sulci indistinct; with a few long setae (Fig. 7F). Antenna short, apparently 2-articulated and with no apical setae (Fig. 7F). Labrum with pair of stout setae (Fig. 7F). Mandibles with inner margin sinuous and darkened. Thoracic nota sclerotized, dark brown, with a row of stout setae on anterior margin. Pro-, meso-, and metanotum with middorsal ecdysial line. Thoracic segments with small pleurites (Fig. 7E). Thoracic legs brown, short and stout, almost the same size of each other. Foreleg with stout setae; tibia with a posteroventral lobe with a spine-like seta (Fig. 7G); femur bearing a spine-like setae; tarsal claw simple. Mid- and hind legs with stout setae on posteroventral margin. Abdomen almost white, with dark brown sclerites. Abdominal segment I with ellipsoid tergite; segments I–V with pair of long, dorsal setae and pair of dorsolateral setae; segment VI with two pairs of long dorsal setae and two pairs of dorsolateral setae; segments VII and VIII with three pairs of dorsal, long setae and two pair of dorsolateral setae; segment IX with sclerotized tergite and several long setae. Abdominal segments I, III, IV, and IX with pair of ventral, long setae; segment II with two pairs of ventral, long setae and globose process on ventrolateral area (Fig. 7E). Anal proleg very short not projecting prominently; with basal sclerite bearing long setae; anal claw simple.
Larval case. Length 1.5–2.0 mm (n = 10). General color white (Figs. 8B and 8C). Constructed with calcareous particles (with no algal filaments added), forming two rigid and lateral valves, poorly closed dorsally and ventrally (Fig. 8C). External surface rugose.
Biology. Larvae were collected on calcareous tufa in a fast flowing river, approximately 10 m wide (Fig. 8A). No pupae were found and adults were not seen active during the day.
Holotype. BRAZIL: Mato Grosso do Sul: Bonito, Rio Formosinho, el. 275 m, 08–13.ix.2013, APM Santos & DM Takiya cols., Malaise trap, male (DZRJ).
Paratypes. Same data as holotype, 3 males (DZRJ), 1 male (MNRJ).
Additional material. Same data as holotype, except 13.ix.2013, manual, 10 larvae (DZRJ), 10 larvae (MNRJ).
Etymology. This species is named in reference to the type locality (Fig. 8), the municipality of Bonito in the state of Mato Grosso do Sul. In Portuguese, the word “bonita” (the feminine form) means “beautiful”.
Remarks. Metrichia bonita sp. nov. has features of the nigritta group: internal pouches between segment V–VI and phallus with 2 spines and an acute process on distal portion. This new species can be easily distinguished from other species in this group based on the shape of inferior appendages, subtrapezoidal in ventral view, with dorsal corners acute and darkened. In addition, inferior appendages have dorsal hooks, which are broad basally and slightly downturned in lateral view. COI sequences showed maximum intraspecific distance of 1.5% and minimum interspecific distance of 21.0% to its closest neighbor, M. itabaiana sp. nov. Although the male genitalia of both species show some superficial resemblance, based on the abdominal modifications and phallic aspect, M. bonita sp. nov. belongs to the nigritta group, whereas M. itabaiana fits better in the campana group.
Larvae of Metrichia bonita sp. nov. are very similar to those previously described or illustrated, including M. nigritta, M. juana, and unassociated larvae illustrated by Botosaneanu & Flint Jr (1982) from Venezuela and Ecuador; and by Pes, Hamada & Nessimian (2005) from Brazil. Actually, main differences seem to be the shape and the material of larval cases. In this respect, larvae of Metrichia bonita sp. nov. are unusual and easily recognized by having their case made entirely of calcareous particles, without typical algal elements (Fig. 8).
Paprocki, Holzenthal & Cressa (2003) discussed the role of Smicridea travertinera Paprocki, Holzenthal & Cressa, 2003 in calcareous tufa formation (travertine). According to these authors, larvae of that species interfere in the deposition and erosion of the calcareous substrate by their net-building activities (Paprocki, Holzenthal & Cressa, 2003). Cyanobacteria and diatoms are known to participate in travertine formation, but the role played by macroinvertebrates is poorly understood (Drysdale, 1998; Drysdale, 1999). It is possible that cases of Metrichia bonita sp. nov. are impregnated passively with calcareous particles, but as commented by Drysdale (1999) for other aquatic insects, they could be important in travertine biogenesis by producing new nucleation sites or eroding other ones. Metrichia bonita sp. nov. is the only microcaddisfly known to inhabit (Fig. 8) and build cases with calcareous tufa so far.
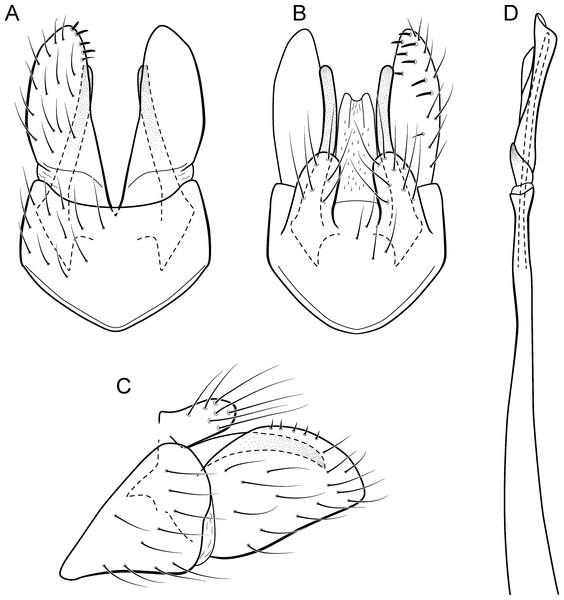
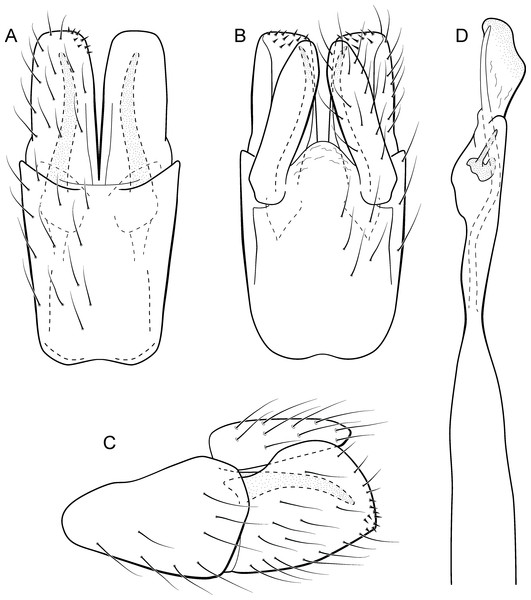
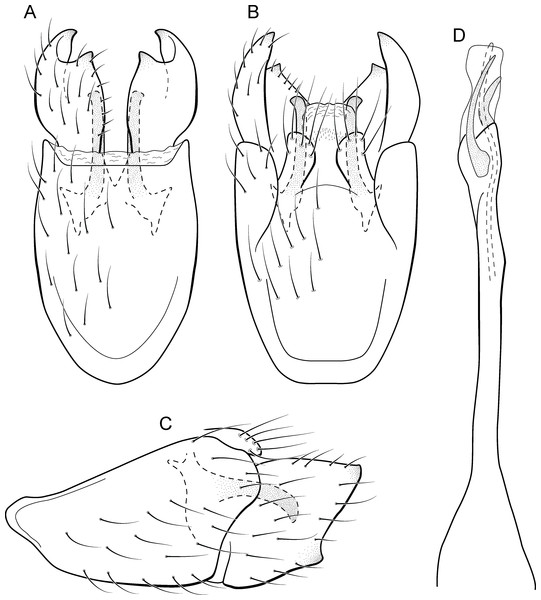
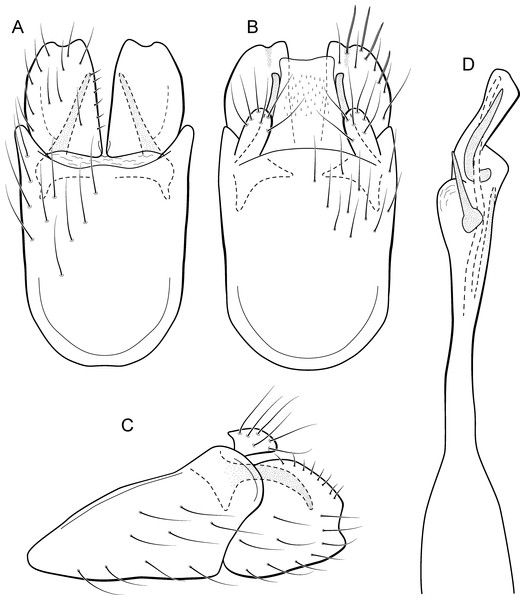
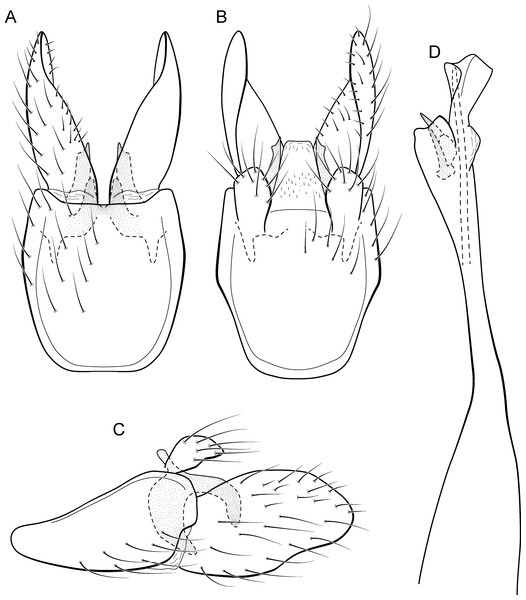
Metrichia bracui sp. nov.
urn:lsid:zoobank.org:act:07B44840-CAB0-4BBE-BD01-B62E885BE418
(Fig. 9)
Figure 9: Metrichia bracui sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 1.8–2.2 mm (n = 3). General color, in alcohol, light brown. Head with no modifications. Ocelli 3. Antenna simple, 18-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdomen without modifications. Ventromesal process on segment VII absent. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subpentagonal in ventral view (Fig. 9A); in lateral view narrower anteriorly than posteriorly (Fig. 9C). Inferior appendage covered by long setae and with scale-like setae, subrectangular in ventral view (Fig. 9A); in lateral view, rounded, apex rounded (Fig. 9C). Dorsal hook long, more than half length of inferior appendage; in lateral view, downturned (Fig. 9C). Preanal appendage elongate and bearing very long setae (Fig. 9B). Subgenital plate apparently absent. Tergum X membranous and with shallow U-shaped incision (Fig. 9B). Phallus tubular, elongate and slender, slightly constricted mesally; without spines, but with a sclerotized process arising from a subapical constriction; apex rounded and folded; ejaculatory duct sclerotized, straight and not protruding apically (Fig. 9D).
Holotype. BRAZIL: Rio de Janeiro: Angra dos Reis, Rio Bracuí, , el. 75 m, 10–11.v.2002, JL Nessimian col., light trap, male (DZRJ).
Paratypes. Same data as holotype, 2 males (MNRJ). Rio de Janeiro: Parque Nacional do Itatiaia, Córrego do Maromba, , el. 1,250 m, 04.iv.15, APM Santos & DM Takiya cols., Malaise trap, 4 males (DZRJ).
Etymology. The species is named in allusion to the river where the holotype was collected.
Remarks. This new species can be assigned to the patagonica group because of the absence of curved spines of the phallus. The general aspect of the male genitalia resembles M. patagonica (Flint, 1983), M. pernambucana, and M. pseudopatagonica Bueno-Soria & Holzenthal, 2003. Metrichia bracui sp. nov. differs from these species and others in the group specially by the phallus bearing a sclerotized process on a constricted region.
The four COI sequences generated for M. bracui sp. nov. were from specimens collected in a locality at Parque Nacional do Itatiaia, Rio de Janeiro, Brazil. The highest pairwise intraspecific divergence between sequences was 0.4%, and the lowest interspecific divergence was 21.4% between M. bracui sp. nov. and Angrisanoia cebollati (Angrisano, 1995). Until now, there have been no formal studies on the relationships among species or genera included in Ochrotrichiinae. However, to infer phylogenetic hypothesis for the entire subfamily is beyond the scope of this work, and also much more data and taxa sampling are necessary to generate robust hypotheses.
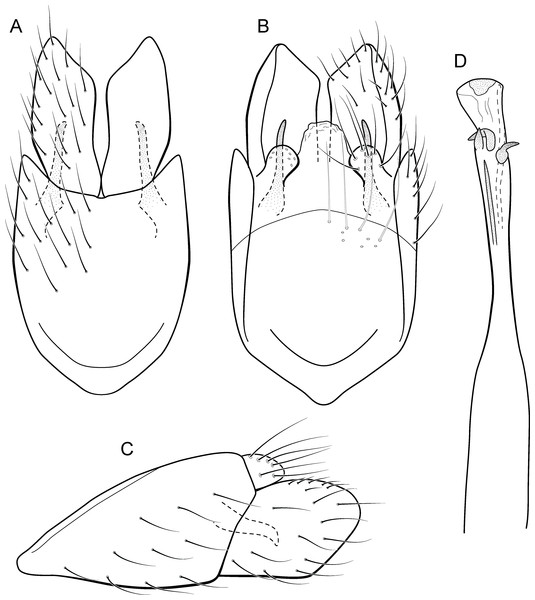
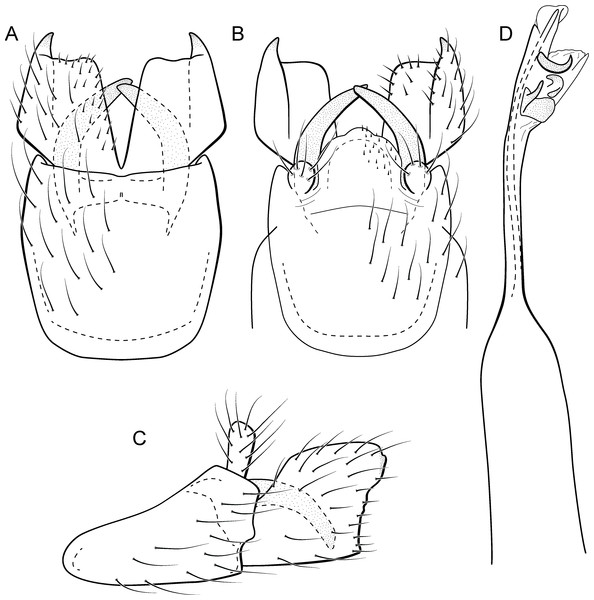
Metrichia caraca sp. nov.
urn:lsid:zoobank.org:act:34F912BC-1069-433E-AF16-A1B19D7FA622
(Fig. 10)
Figure 10: Metrichia caraca sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 2.5–3.0 mm (n = 5). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Antenna simple, 18-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment VI bearing brush of very long setae dorsolateraly; segment VII bearing brush of very long setae dorsolateraly. Ventromesal process on segment VII absent. Segment VIII shorter ventrally than dorsally and bearing brush of long setae dorsally. Male genitalia. Segment IX reduced dorsally; sternum subrectangular, with anterior margin rounded (Fig. 10A); in lateral view narrower anteriorly than posteriorly (Fig. 10C). Inferior appendage covered by long setae, subtrapezoidal in ventral view (Fig. 10A), apex oblique and projected mesad into a large process bearing a stout spine-like setae. Dorsal hook short and straight; in lateral view, truncate and broader apically (Fig. 10C). Preanal appendage short, truncate and bearing very long setae (Fig. 10B). Subgenital plate apparently absent. Tergum X membranous and truncate (Fig. 10B). Phallus tubular, elongate and slender, slightly constricted mesally; with two curved subapical spines, one short and another long; apex emarginate; ejaculatory duct sclerotized and not protruding apically (Fig. 10D).
Holotype. BRAZIL: Minas Gerais: Catas Altas, RPPN Santuário do Caraça, Ribeirão Caraça, 11–13.vi.2013, ML Monné & JP Botero cols., Malaise trap, male (DZRJ).
Paratypes. Same data as holotype, 3 males (DZRJ). Minas Gerais: São Roque de Minas, Parque Nacional da Serra da Canastra, Fazenda Velha, Córrego dos Pombos, , el. 997 m, 02.iv.2014, JL Nessimian, ALH Oliveira, LL Dumas & SP Gomes, light trap cols., 1 male (MNRJ).
Etymology. This species is named in reference to the stream where type specimens were collected.
Remarks. This new species has very distinctive male genitalia. Based on the dorsoapically produced inferior appendages, it resembles M. lenophora (Flint, 1991). However, M. caraca sp. nov. is easily recognized by the obliquely truncate and mesad directed process of inferior appendages and dorsal hook, in lateral view, with apex broad and truncate.
COI distances within this species reached only 1.1% and the lowest interspecific distance (21.7%) was found between specimens of M. caraca sp. nov. and M. acuminata sp. nov., which are very distinct based on morphological features.
Metrichia circuliforme sp. nov.
urn:lsid:zoobank.org:act:E539EF55-F963-433C-BB25-C9177339ED54
(Fig. 11)
Figure 11: Metrichia circuliforme sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 2.5–2.7 mm (n = 4). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Antenna simple, 18-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment VI bearing brush of very long setae dorsolateraly; segment VII bearing a brush of very long setae dorsolateraly. Ventromesal process on segment VII present. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subpentagonal (Fig. 11A); in lateral view narrower anteriorly than posteriorly (Fig. 11C). Inferior appendage short, covered by long setae, subrectangular in ventral view (Fig. 11A); in lateral view, rounded (Fig. 11C), apex slightly truncate and bearing short spine-like setae. Dorsal hook long, almost reaching the inferior appendage apex; in lateral view, downturned (Fig. 11C). Preanal appendage elongate, but shorter than inferior appendage, and bearing very long setae (Fig. 11B). Subgenital plate apparently absent. Tergum X membranous and truncate (Fig. 11B). Phallus tubular, elongate and slender, slightly constricted mesally and with a median process; with two short subapical spines; apex rounded and sclerotized; ejaculatory duct sclerotized, straight and not protruding apically (Fig. 11D).
Holotype. BRAZIL: Rio de Janeiro: Itatiaia, Rio das Pedras, Cachoeira de Deus, , el. 689 m, 06.iii.2008, JL Nessimian, LL Dumas & MR de Souza cols., light trap, male (DZRJ).
Paratypes. Same data as holotype, except Rio das Pedras, , el. 706 m, 06.iii.2008, LL Dumas, JL Nessimian & MR de Souza cols., light trap, 1 male (DZRJ), 1 male (MNRJ); Parque Nacional do Itatiaia, Córrego Simon, , el. 1,033 m, 15.iv.07, LL Dumas, APM Santos, N Ferreira-Jr. & JL Nessimian cols., light trap, 1 male (DZRJ).
Etymology. The new species name is an allusion to the rounded and simple inferior appendages, derived from the Latin, “circuli-” and “form” meaning “rounded shape”.
Remarks. This species has simple male genitalia and abdomen with only brushes of long setae on segments VI and VII. General aspect of the male genitalia is similar to M. riva (Bueno-Soria, 1983) and M. quadrata (Flint Jr, 1972), particularly their inferior appendages short and subrectangular and phallus with two subapical spines. However, M. circuliforme sp. nov. can be easily distinguished from M. quadrata by the absence of internal sacs in the abdomen. It can be distinguished from M. riva by the elongate preanal appendages and phallus with subequal hook spines subapically.
We obtained seven COI sequences for M. circuliforme sp. nov. and although all of them came from specimens collected at the same locality, intraspecific divergences were relatively high, reaching 3.5%. Besides that, GMYC estimated two species for these sequences instead of one. Re-analysis of the morphology of these specimens did not reveal any conspicuous variation that could justify splitting this species into two taxonomic groups. Compared to other studies using DNA barcodes of caddisflies, this genetic distance is still low, for example Pauls et al. (2010) found intraspecific divergences (K2P distance) up to 5.9% for Chilean Smicridea. Zhou et al. (2011) found even higher intraspecific distances among caddisflies, reaching up to 14%. GMYC is known to be more sensitive to geographic range coverage and/or other sampling schemes, resulting in oversplitting (Lohse, 2009; Talavera, Dincă & Vila, 2013). Therefore, we consider M. circuliforme sp. nov. as a robust species based on morphology as well as based on barcode divergences. Minimum interspecific COI distances of M. circuliforme sp. nov. to M. curta sp. nov. were 18.4%, and again, these two species are very distinct based on morphological features and apparently are not even closely related to each other (Fig. 2).
Metrichia curta sp. nov.
urn:lsid:zoobank.org:act:7EC0620B-D6F2-409C-8351-79A3D3FB77C5
(Fig. 12)
Figure 12: Metrichia curta sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 2.4–2.5 mm (n = 7). General color, in alcohol, light brown. Head with no modifications. Ocelli 3. Antenna simple, 18-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdomen without modifications. Ventromesal process on segment VII present. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subpentagonal (Fig. 12A); in lateral view, narrower anteriorly than posteriorly (Fig. 12C). Inferior appendage covered by long setae, subtrapezoidal in ventral view (Fig. 12A); in lateral view, subtriangular (Fig. 12C), apex rounded. Dorsal hook long, more than half length of inferior appendage; in lateral view, with apex slightly broader, downturned, and truncate (Fig. 12C). Preanal appendage elongate, but shorter than inferior appendage, and bearing very long setae (Fig. 12B). Subgenital plate apparently absent. Tergum X membranous and rounded (Fig. 12B). Phallus tubular, elongate and slender, slightly constricted mesally; with a stout subapical spine; apex rounded and folded; ejaculatory duct sclerotized, straight and protruding apically (Fig. 12D).
Holotype. BRAZIL: Rio de Janeiro: Itatiaia, Rio das Pedras, , el. 706 m, 06.iii.2008, LL Dumas, JL Nessimian & MR de Souza cols., light trap, male (DZRJ).
Paratypes. Same data as holotype, 3 males (DZRJ), 3 males (MNRJ).
Etymology. The specific name is a reference to the very short inferior appendage; in Portuguese “curta” means “short.”
Remarks. Based on the absence of modifications on abdominal segments, this new species can be assigned to the aberrans group. Its phallus is similar to that of M. amplitudinis Bueno-Soria & Holzenthal, 2003, with a lonjg spine and an apical flap. The new species can be distinguished by the triangular inferior appendages in lateral view and phallus with a strongly curved spine subapically. Metrichia amplitudinis and M. curta sp. nov. share the widened dorsal hook, but in the new species this structure is only slightly wider and also truncate in lateral view. Maximum intraspecific divergence of COI sequences was 1.5% for M. curta sp. nov. and minimum interspecific was to M. circuliforme, as mentioned above.
Metrichia farofa sp. nov.
urn:lsid:zoobank.org:act:BC4FF095-32BE-46A9-BEAB-28854E2F5BC7
(Fig. 13)
Figure 13: Metrichia farofa sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 1.8–2.1 mm (n = 27). General color, in alcohol, light brown. Head with no modifications. Ocelli 3. Antenna simple, 18-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdomen without modifications; segment VII bearing specialized setae dorsally. Ventromesal process on segment VII absent. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subrectangular (Fig. 13A); in lateral view narrower anteriorly than posteriorly (Fig. 13C). Inferior appendage covered by long setae, subrectangular in ventral view (Fig. 13A); in lateral view, subrectangular (Fig. 13C), apex slightly truncate and bearing short spine-like setae. Dorsal hook long, almost reaching the inferior appendage apex; in lateral view, downturned (Fig. 13C). Preanal appendage elongate, as long as inferior appendage, and bearing very long setae (Fig. 13B). Subgenital plate apparently absent. Tergum X membranous and rounded (Fig. 13B). Phallus tubular, elongate and slender, slightly constricted mesally; with a stout subapical spine; apex rounded and sclerotized; ejaculatory duct sclerotized, sinuous, and protruding apically (Fig. 13D).
Holotype. BRAZIL: Minas Gerais: Jaboticatubas, Parque Nacional da Serra do Cipó, Cachoeira da Farofa, , el. 811 m, 23.iv.2010, APM Santos & DM Takiya cols., manual, male (DZRJ).
Paratypes. Same data as holotype, 18 males (DZRJ), 8 males (MNRJ), 5 males (MZUFBA); same data, except Ribeirão Mascates, , el. 820 m, 09–11.xii.2011, APM Santos & DM Takiya cols., manual, 84 males (DZRJ).
Etymology. This new species is named in reference to the waterfall where specimens were collected.
Remarks. Due to absence of pouches in abdominal segments, Metrichia farofa sp. nov. can also be included in the aberrans group. However, the new species has a single subapical spine in phallus, like those species included in the exclamationis group. The new species can be easily distinguished from all other Metrichia species by the very long preanal appendages, reaching apices of inferior appendages in dorsal and lateral views.
Although more than 10 specimens of M. farofa sp. nov. were submitted to DNA extraction and many attempts of COI amplification via PCR were conducted, we were not able to obtain sequences of this species, even using recent material collected after 2013.
Metrichia forceps sp. nov.
urn:lsid:zoobank.org:act:8F25D006-D59B-4C83-8CDE-2398368917AD
(Fig. 14)
Figure 14: Metrichia forceps sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 2.7–3.0 mm (n = 2). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Antenna simple, 23-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur with small acute apical process. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment VII bearing internal pouches in anterior area. Ventromesal process on segment VII absent. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subpentagonal (Fig. 14A); in lateral view, narrower anteriorly than posteriorly (Fig. 14C). Inferior appendage covered by long setae, subrectangular in ventral view (Fig. 14A); in lateral view, with posterior margin excavated and with two acute and sclerotized process (Fig. 14C). Dorsal hook long, almost reaching the inferior appendage apex; in lateral view, downturned (Fig. 14C). Preanal appendage short, rounded and bearing very long setae (Fig. 14B). Subgenital plate apparently absent. Tergum X sclerotized, deeply notched mesally, forming lateral curved processes (Fig. 14B). Phallus tubular, elongate and slender, slightly constricted mesally; with two short subapical spines; apex rounded and folded; ejaculatory duct sclerotized, straight and protruding apically (Fig. 14D).
Holotype. BRAZIL: Paraná: Céu Azul, Parque Nacional do Iguaçu, Rio Azul, , el. 510 m, 6–8 ix.2012, APM Santos, DM Takiya, ALH Oliveira, GA Jardim & BHL Sampaio cols., Malaise trap, male (DZRJ).
Paratypes. Same data as holotype, 1 male (MNRJ).
Etymology. The name of this species is in reference to the dorsal hooks of inferior appendages, which in ventral view resemble forceps.
Remarks. This new species belongs to the campana group, sharing the diagnostic internal pouches between segments VI and VII, reduced spines on subapical region of phallus, and the sclerotized and elongate tergum X. Within this group, M. forceps sp. nov. is most similar to M. campana (Flint Jr, 1968), M. similis (Flint Jr, 1968), and M. continentalis (Flint Jr, 1972), particularly by their inferior appendages with excavate posterior margins, forming two pointed processes, one ventral and another dorsal. The new species can be distinguished from the others by its deeply notched tergum X; dorsal hook of inferior appendages elongate and downturned, and phallus apex bearing two small spines and a sclerotized flap surrounding the protruding ejaculatory duct.
Metrichia formosinha sp. nov.
urn:lsid:zoobank.org:act:B8971D9B-7013-4213-970E-50A42CA0D1B7
(Fig. 15)
Figure 15: Metrichia formosinha sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 2.5–2.8 mm (n = 2). General color, in alcohol, dark brown. Head with no modifications. Ocelli 3. Antenna simple, 20-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur with small acute apical process. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment VI with pair of internal pouches in posterodorsal area. Ventromesal process on segment VII present. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subrectangular, with anterior margin rounded (Fig. 15A); in lateral view narrower anteriorly than posteriorly (Fig. 15C). Inferior appendage with peg-like setae, subrectangular in ventral (Fig. 15A) and lateral (Fig. 15C) views; apex with acute corners. Dorsal hook long, almost reaching the inferior appendage apex; in lateral view, with apex slightly broader, almost straight, and truncate (Fig. 15C). Preanal appendage short, rounded and bearing very long setae (Fig. 15B). Subgenital plate apparently absent. Tergum X membranous and rounded (Fig. 15B). Phallus tubular, elongate and slender, slightly constricted mesally; with two curved subapical spines, one short and another one very long; apex rounded and sclerotized; ejaculatory duct sclerotized, sinuous, and not protruding apically (Fig. 15D).
Holotype. BRAZIL: Mato Grosso do Sul: Bonito, Rio Formosinho, el. 275 m, 08–13.ix.2013, APM Santos & DM Takiya cols., Malaise trap, male (DZRJ).
Paratypes. Same data as holotype, 3 males (DZRJ).
Etymology. This species is named in allusion to the river where type specimens were collected.
Remarks. This new species appears to be a member of the campana group because of internal pouches between abdominal segments VI–VII and phallus with two subapical spines. General aspect of the male genitalia of Metrichia formosinha sp. nov. is similar to M. forceps sp. nov., particularly in the inferior appendages with acute corners. However, M. formosinha sp. nov. differs from the latter by dorsal hooks almost straight and capitate (strongly curved and acute in M. forceps sp. nov. and in other species of the campana group) and phallus with a very long subapical spine.
COI sequences of M. formosinha sp. nov. showed intraspecific divergences up to 0.8% and minimum interspecific divergences of 24.9% compared to M. talhada sp. nov.
Metrichia goiana sp. nov.
urn:lsid:zoobank.org:act:8726E665-1093-42EF-A1B6-E06178B2DAD1
(Fig. 16)
Figure 16: Metrichia goiana sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 1.8–2.0 mm (n = 4). General color, in alcohol, dark brown. Head with no modifications. Ocelli 3. Antenna simple, 18-articulated. Maxillary palpus 5-articulated, article IV broad and darkened; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur with small acute apical process. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment V with pair of internal pouches and pair of dorsolateral brushes; segment VI with a transverse sclerotized plate posteriorly on dorsum; segment VII bearing specialized setae dorsally. Ventromesal process on segment VII absent. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subrectangular, with anterior margin rounded (Fig. 16A); in lateral view narrower anteriorly than posteriorly (Fig. 16C). Inferior appendage covered by long setae, elongate and narrow in ventral view (Fig. 16A); in lateral view, rounded (Fig. 16A), apex rounded and bearing a tooth-like projection (Fig. 16A). Dorsal hook short, less than half length of inferior appendage; in lateral view, downturned (Fig. 16A). Preanal appendage short and bearing very long setae (Fig. 16B). Subgenital plate apparently absent. Tergum X membranous and truncate (Fig. 16B). Phallus tubular, elongate and slender, slightly constricted mesally; with two long, curved, subapical spines; apex ending into two sclerotized and keel shaped processes; ejaculatory duct sclerotized, straight and not protruding apically (Fig. 16D).
Holotype. BRAZIL: Goiás: Alto Paraíso de Goiás, Rio Bartolomeu tributary, , el. 1,165 m, 22–25.iii.2013, APM Santos & DM Takiya cols., Malaise trap, male (DZRJ).
Paratypes. Same data as holotype, 2 males (MNRJ), 1 male (DZRJ).
Etymology. The species is named in reference to Goiás State. “Goiana” is a Portuguese adjective for people from Goiás.
Remarks. This is another member of nigritta group. Male genitalia of this species are similar to M. potosina Bueno-Soria, 2002 and M. ubajara sp. nov., due to rounded and elongate inferior appendages in lateral view. This new species differs from M. ubajara sp. nov. by the presence of an apical tooth on inferior appendages, also present in M. potosina. Metrichia goiana sp. nov. can be distinguished from M. potosina by the two long subapical spines on phallus, whereas M. potosina has three.
Metrichia itabaiana sp. nov.
urn:lsid:zoobank.org:act:1C902E75-7ECD-4875-A680-6440A3E5E9E9
Figure 17: Metrichia itabaiana sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 1.8–2.1 mm (n = 3). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Antenna simple, 20-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment V ventrally with a mesal brush of long setae; segment VI with tergum as a sclerotized triangular plate surrounded by specialized setae (Fig. 26B), internally with pair of internal pouches; segment VII bearing specialized setae ventrally and dorsally (Fig. 26B). Ventromesal process on segment VII present. Segment VIII shorter ventrally than dorsally and bearing a brush of long setae dorsally. Male genitalia. Segment IX reduced dorsally; sternum subpentagonal (Fig. 17A); in lateral view narrower anteriorly than posteriorly (Fig. 17C). Inferior appendage short, covered by long setae (Fig. 17A); in lateral view, subtrapezoidal (Fig. 17C), apex excavated and with two acute and sclerotized process (Figs. 17A and 17B). Dorsal hook short, almost half length of inferior appendage; in lateral view, slightly downturned (Fig. 17C). Preanal appendage elongate, but shorter than inferior appendage, and bearing very long setae (Fig. 17B). Subgenital plate apparently absent. Tergum X membranous and truncate (Fig. 17B). Phallus tubular, elongate and slender, slightly constricted mesally; with two long, curved, subapical spines; apex truncate and slightly sclerotized; ejaculatory duct sclerotized, sinuous, and protruding apically (Fig. 17D).
Holotype. Sergipe: Areia Branca, Parque Nacional da Serra de Itabaiana, Rio dos Negros, , el. 208 m, 17.vi.2014, APM Santos, DM Takiya & WRM Souza cols., light trap, male (DZRJ).
Paratypes. Same data as holotype, 1 male (DZRJ), 1 male (MZUFBA); same data, except Riacho Água Fria, , el. 196 m, 17–19.vi.2014, APM Santos, DM Takiya, WRM Souza cols., Malaise trap, 2 males (MNRJ). Goiás: Alto Paraíso, Rio Bartolomeu tributary, , el. 1,165 m, 22–25.iii.2013, APM Santos & DM Takiya cols., Malaise trap, 1 male (DZRJ).
Etymology. This species is named in reference to Serra de Itabaiana, Sergipe, where the holotype was collected.
Remarks. This new species appears to be a member of the campana group because of internal pouches between abdominal segments VI and VII and pair of long subapical spines on phallus, but it lacks the acute process on the mesal area of phallus. Male genitalia of M. itabaiana sp. nov. resemble those of M. campana and M. vulgaris sp. nov., particularly, in the excavated inferior appendages, with acute and darkened corners. However, the new species can be recognized by very long curved subapical spines on phallus and subtrapezoidal aspect of inferior appendages in lateral view.
Only two COI sequences were obtained for M. itabaiana sp. nov., one from Sergipe (Northeastern Brazil) and another from Goiás (Centralwestern Brazil). The COI divergence between these two samples was 1.9% and minimum interspecific distance was 19.4% in relation to M. rafaeli sp. nov., which belongs to a different species group based on morphological features.
Metrichia longissima sp. nov.
urn:lsid:zoobank.org:act:F87C549F-6F84-4466-AFCE-940729F32F46
Figure 18: Metrichia longissima sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 2.5–2.7 mm (n = 2). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Antenna simple, 18-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur with small acute apical process. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment IV with dorsal area expanded posteriorly bearing stout setae; segment VI with stout and striate setae (Fig. 26C); segment VII with stout and striate setae (Fig. 26C). Ventromesal process on segment VII absent. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subpentagonal (Fig. 18A); in lateral view narrower anteriorly than posteriorly (Fig. 18C). Inferior appendage bearing scale-like setae, very elongate; in ventral view, curved inward apically (Fig. 18A); in lateral view, tapering to a rounded apex (Fig. 18C). Dorsal hook short and straight; in lateral view, slightly downturned (Fig. 18C). Preanal appendage elongate, but shorter than half length of inferior appendage, and bearing stout and striate setae (Fig. 18B). Subgenital plate apparently absent. Tergum X membranous and truncate (Fig. 18B). Phallus tubular, elongate and slender, slightly constricted mesally, with a median process; with two long, curved, subapical spines; apex rounded and sclerotized; ejaculatory duct sclerotized and protruding apically (Fig. 18D).
Holotype. BRAZIL: Rio de Janeiro: Itatiaia, Rio Palmital, , el. 637 m, 07.iii.2008, LL Dumas, JL Nessimian & MR de Souza cols., light trap, male (DZRJ).
Paratype. Brazil: Rio de Janeiro: Teresópolis, Parque Nacional da Serra dos Órgãos, Rio Paquequer, , el. 1,100 m, 15–18.ix.2011, APM Santos, DM Takiya, BM Vasconcelos & RA Carvalho cols., Malaise trap, 1 male (MNRJ).
Etymology. The species name is an allusion to the elongate inferior appendages, unusual for Metrichia species.
Remarks. The new species is most similar to M. sesquipedalis Bueno-Soria & Holzenthal, 2003, sharing with it very long inferior appendages with very short dorsal hooks. The new species is easily distinguished from the latter by their internal pouches in the male abdominal segment VI and phallus with only two subapical spines (three in M. sesquipedalis).
Two COI sequences were generated for M. longissima sp. nov., one from a specimen from Itatiaia and the other from Teresópolis in Rio de Janeiro State, localities in distinct mountain ranges, Serra da Mantiqueira and Serra do Mar, respectively. The genetic distance between sequences of M. longissima sp. nov. was 0.4%. The minimum interspecific distance was found to M. itabaiana sp. nov. with 21.5% divergence.
Metrichia peluda sp. nov.
urn:lsid:zoobank.org:act:E1B7E1AE-5751-4D10-9B07-47A8CF849C7F
Figure 19: Metrichia peluda sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 2.7–3.0 mm (n = 3). General color, in alcohol, dark brown. Head with no modifications. Ocelli 3. Antenna simple, 18-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment V with dorsolateral brushes of long setae; segment VI with dorsolateral brushes of long setae (Fig. 26D). Ventromesal process on segment VII present. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subrectangular, with anterior margin rounded (Fig. 19A); in lateral view narrower anteriorly than posteriorly (Fig. 19C). Inferior appendage covered by long setae, subtrapezoidal in ventral view (Fig. 19A); apex excavated; in lateral view, rounded (Fig. 19C). Dorsal hook short, almost half length of inferior appendage; in lateral view, slightly downturned (Fig. 19C). Preanal appendage short and bearing very long setae (Fig. 19B). Subgenital plate apparently absent. Tergum X membranous and truncate (Fig. 19B). Phallus tubular, elongate and slender, slightly constricted mesally; with two curved subapical spines, one short and another long; apex rounded and folded; ejaculatory duct sclerotized, straight and protruding apically (Fig. 19D).
Holotype. BRAZIL: Rio de Janeiro: Itatiaia, 1st order tributary of Rio Palmital, , el. 584 m, 07.iii.2008, JL Nessimian, LL Dumas & MR de Souza cols., light trap, male (DZRJ).
Paratypes. Same data as holotype, 1 male (MNRJ); same data, except Rio Palmital, , el. 637 m, 07.iii.2008, LL Dumas, JL Nessimian & MR de Souza cols., light trap, 4 males (DZRJ).
Etymology. The name of this species refers to dense brushes of setae on the dorsal area of the male abdomen. In Portuguese “peluda” means “hairy.”
Remarks. Modifications on male abdominal segments V, VI, and VII suggest that this new species belongs to the campana group. The general aspect of inferior appendages is somewhat similar to M. forceps sp. nov. and M. formosinha sp. nov., which are excavate posteriorly. However, M. peluda sp. nov. is readily identified by the dense brushes of setae on the dorsum of abdominal segments V, VI, and VII. Besides, the male genitalia of this new species differ from those described for M. forceps sp. nov. and M. formosinha sp. nov. by the rounded corners of inferior appendages instead of acute and by phallus with two subapical spines with unequal sizes.
Metrichia rafaeli sp. nov.
urn:lsid:zoobank.org:act:CBCADBB8-2C79-49AB-8345-CC1E6FA2AEE9
(Fig. 20)
Figure 20: Metrichia rafaeli sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 2.0–2.5 mm (n = 4). General color, in alcohol, dark brown. Head with no modifications. Ocelli 3. Antenna simple, 20-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment V with pair of internal pouches; segment VI with pair of internal pouches and pair of lateral external sacs with specialized setae; segment VII bearing specialized setae dorsally. Ventromesal process on segment VII present. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subrectangular, with anterior margin rounded (Fig. 20A); in lateral view narrower anteriorly than posteriorly (Fig. 20C). Inferior appendage covered by long setae, short and rounded, with apex slightly excavated (Fig. 20A); in lateral view, rounded (Fig. 20C). Dorsal hook long, more than half length of inferior appendage; in lateral view, downturned (Fig. 20C). Preanal appendage elongate, but shorter than half length of inferior appendage, and bearing stout and striate setae (Fig. 20B). Subgenital plate apparently absent. Tergum X membranous and truncate (Fig. 20B). Phallus tubular, elongate and slender, slightly constricted mesally, with a median process; with two long, curved, subapical spines, and a membranous lobe; apex rounded and sclerotized; ejaculatory duct sclerotized, sinuous, and not protruding apically (Fig. 20D).
Holotype male. BRAZIL: Ceará: Ubajara, Parque Nacional de Ubajara, Rio das Minas, , el. 524, 17–18.ii.2013, DM Takiya, JA Rafael, RR Cavichioli & APM Santos cols., Malaise trap (CZMA).
Paratypes. Same data as holotype, 1 male (MZUFBA); same data, except Rio das Minas, , el. 420 m, 20–23.iv.2012, F Limeira-de-Oliveira et al.cols., Malaise trap, 1 male (CZMA); same data, except 14–16.ii.2013, DM Takiya, JA Rafael, RR Cavichioli & APM Santos cols., 1 male (DZRJ).
Etymology. This species is named in honor of the Brazilian entomologist Dr. José Albertino Rafael (INPA), who has collected many interesting caddisflies, including some species described here.
Remarks. This new species belongs to the nigritta group due to internal pouches between abdominal segments V and VI and long and acute process on phallus. The male genitalia of M. rafaeli sp. nov. are more similar to M. magna Bueno-Soria & Holzenthal, 2003 with short and simple inferior appendages. However, the new species can be easily distinguished from the latter and other Metrichia species in this group by the posterior margin of inferior appendages slightly excavated and very long subapical spines of phallus.
Although specimens with barcode sequences of M. rafaeli sp. nov. were collected at the same locality, haplotypes were not identical having intraspecific divergence of 0.4%. The lowest interspecific distance was 19.4% when compared with M. itabaiana sp. nov.
Metrichia simples sp. nov.
urn:lsid:zoobank.org:act:05933E6E-A6FF-49DE-982E-54B263830D10
Figure 21: Metrichia simples sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 2.1–2.2 mm (n = 2). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Antenna simple, 19-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment IV with dorsal area expanded posteriorly bearing stout setae; segment VI bearing very long setae laterally, with a brush of short setae covered dorsally by a triangular plate (Fig. 26E); segment VII with a brush of short setae dorsally (Fig. 26E). Ventromesal process on segment VII present. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subpentagonal (Fig. 21A); in lateral view narrower anteriorly than posteriorly (Fig. 21C). Inferior appendage covered by long setae, with apex obliquely truncate; subtrapezoidal in ventral view (Fig. 21A); in lateral view, subtrapezoidal (Fig. 21C). Dorsal hook short, almost half length of inferior appendage; in lateral view, slightly downturned (Fig. 21C). Preanal appendage short, rounded and bearing very long setae (Fig. 21B). Subgenital plate apparently absent. Tergum X membranous and rounded (Fig. 21B). Phallus tubular, elongate and slender, slightly constricted mesally; with four subapical spines, three short and one long and straight; apex rounded with a small sclerite; ejaculatory duct sclerotized and not protruding apically (Fig. 21D).
Holotype. BRAZIL: Paraná: Céu Azul, Parque Nacional do Iguaçu, Rio Azul, , el. 510 m, 6–8 ix.2012, APM Santos, DM Takiya, ALH Oliveira, GA Jardim & BHL Sampaio cols., Malaise trap, male (DZRJ).
Paratypes. Same data as holotype, 1 male (MNRJ).
Etymology. This species is named in reference to the simple aspect of the male genitalia and abdomen, without modifications and processes seen in other Metrichia species.
Remarks. This species can be assigned to the campana group because of pouches in abdominal segments VI and VII. The new species shares the general aspect of the genitalia with M. quadrata, particularly, in the quadrangular or rectangular shape of inferior appendages and the short subapical spines on phallus. Metrichia simples sp. nov. can be recognized by the short dorsal hook, reaching up to midlength of inferior appendages (subequal to inferior appendages in M. quadrata), obliquely truncate apex of the inferior appendages, and strongly curved spines on phallus.
Metrichia talhada sp. nov.
urn:lsid:zoobank.org:act: 5456FEB8-5193-46DD-A10F-9FFADFCB59EC
(Fig. 22)
Figure 22: Metrichia talhada sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 1.8–2.0 mm (n = 10). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Antenna simple, 18-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur with small acute apical process. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment V with pair of internal pouches and pair of dorsolateral brushes; segment VI with dorsolateral brushes of long setae; segment VII bearing specialized setae dorsally. Ventromesal process on segment VII absent. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subpentagonal (Fig. 22A); in lateral view, narrower anteriorly than posteriorly (Fig. 22C). Inferior appendage elongate, apex rounded and bearing a tooth-like projection; with a deep C-shaped notch in ventral view (Fig. 22A); in lateral view, with an acute projection (Fig. 22C). Dorsal hook short, less than half length of inferior appendage (Fig. 22A); in lateral view, downturned. Preanal appendage short, rounded and bearing very long setae (Fig. 22B). Subgenital plate apparently absent. Tergum X membranous and truncate (Fig. 22B). Phallus tubular, elongate and slender, slightly constricted mesally, with a median process; with two curved subapical spines, one short and another long; apex rounded and sclerotized; ejaculatory duct sclerotized, straight and protruding apically (Fig. 22D).
Holotype. BRAZIL: Alagoas: Quebrangulo, Reserva Biológica de Pedra Talhada, Rio Caranguejo, , el. 550 m, 19–28.vi.2014, APM Santos, DM Takiya, WRM Souza cols., Malaise trap, male (DZRJ).
Paratypes. Same data as holotype, 8 males (DZRJ), 7 males (MZUFBA).
Etymology. This species is named in reference to its type locality, the Reserva Biológica de Pedra Talhada.
Remarks. The new species belongs to the nigritta group because of their internal pouches between abdominal segments V and VI and phallus with two subapical curved spines and an acute process near mesal area. Metrichia talhada sp. nov. shares the apical tooth on inferior appendages with M. potosina, M. goiana sp. nov., and Metrichia tere sp. nov. From M. potosina and M. goiana sp. nov., it is easily distinguished by the shape of the inferior appendages, with a deep C-shaped notch on ventral margin and subapical tooth stout and slightly truncate in lateral view, and subapical spines on phallus, one long and another short. Although the male genitalia of M. talhada sp. nov. are very similar to M. tere sp. nov., these two species can be separated by the shape of inferior appendages teeth, each slightly truncate and subapical in the former and acute and apical in the later species.
Sequences of M. talhada sp. nov. showed 0.0% of divergence. Morphological similarity between this species and its sister (M. tere sp. nov.) had a relatively low genetic divergence of 12.6%, the lowest interspecific distance found in our sampling. In addition to minor but stable differences in genital structures, all molecular analyses with COI sequences (NJ, ABGD, GMYC) corroborate the distinction between M. talhada sp. nov. and M. tere sp. nov., which are formally described here as different species.
Metrichia tere sp. nov.
urn:lsid:zoobank.org:act:21376F9C-6308-47BA-ADB8-A42848AD8FB5
(Fig. 23)
Figure 23: Metrichia tere sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 1.9–2.1 mm (n = 10). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Antenna simple, 18-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur with small acute apical process. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment V with pair of internal pouches and pair of dorsolateral brushes; segment VI with dorsolateral brushes of long setae; segment VII bearing specialized setae dorsally. Ventromesal process on segment VII absent. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subpentagonal (Fig. 23A); in lateral view, narrower anteriorly than posteriorly (Fig. 23C). Inferior appendage elongate, apex with an acute projection; with a deep C-shaped notch in ventral view (Fig. 23A); in lateral view, with an acute projection (Fig. 23C). Dorsal hook short, less than half length of inferior appendage; in lateral view, downturned (Fig. 23C). Preanal appendage short, rounded and bearing very long setae (Fig. 23B). Subgenital plate apparently absent. Tergum X membranous and truncate (Fig. 23B). Phallus tubular, elongate and slender, slightly constricted mesally, with a median process; with two curved subapical spines, one short and another long; apex rounded and sclerotized; ejaculatory duct sclerotized, straight and protruding apically (Fig. 23D).
Holotype. BRAZIL: Rio de Janeiro: Teresópolis, Parque Nacional da Serra dos Órgãos, Rio Paquequer, , el. 1,100 m, 15–18.ix.2011, APM Santos, DM Takiya, BM Vasconcelos & RA Carvalho cols., Malaise trap, male (DZRJ).
Paratypes. Same data as holotype, 9 males (MNRJ), 19 males (DZRJ).
Etymology. This species is named in reference to the city of Teresópolis (= “City of Teresa”), affectionately called as “Terê”. The city was named in honor of Teresa Cristina, Brazilian Empress from 1843 to 1889, wife of Dom Pedro II.
Remarks. This new species is very similar to the preceding one, also belonging to the nigritta group. Metrichia tere sp. nov. can be distinguished from M. talhada sp. nov. by inferior appendages with an apical acute projection, whereas in M. talhada sp. nov. inferior appendages have a subapical tooth, which is more truncate than acute.
Molecular data agree with the morphological distinction of M. tere sp. nov., as commented on above. Intraspecific K2P divergence among specimens sampled (n = 3) of this species was 0.0%, and as mentioned in the previous description, M. talhada sp. nov. and M. tere sp. nov. showed the lowest observed interspecific distance (12.6%).
Metrichia ubajara sp. nov.
urn:lsid:zoobank.org:act:68FD2A24-BC85-42A3-9007-96A42657DAE3
(Fig. 24)
Figure 24: Metrichia ubajara sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Adult male. Length 2.0–2.7 mm (n = 11). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Antenna simple, 18-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment V with pair of internal pouches and median internal plate in posterior region; with specialized setae on dorsum; segment VI with pair of internal pouches in posterodorsal area. Ventromesal process on segment VII absent. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subrectangular (Fig. 24A); in lateral view, narrower anteriorly than posteriorly (Fig. 24C). Inferior appendage covered by long setae, apex rounded; elongate and narrow in ventral view (Fig. 24A); in lateral view, rounded (Fig. 24C). Dorsal hook short, less than half length of inferior appendage; apex downturned; basally with a wide and sclerotized projection; in lateral view, C-shaped (Fig. 24C). Preanal appendage elongate, but shorter than half length of inferior appendage, and bearing stout and striate setae (Fig. 24B). Subgenital plate apparently absent. Tergum X membranous and truncate (Fig. 24B). Phallus tubular, elongate and slender, slightly constricted mesally; with two long, curved, subapical spines; apex rounded and folded; ejaculatory duct sclerotized, sinuous, and protruding apically (Fig. 24D).
Holotype. BRAZIL: Ceará: Ubajara, Parque Nacional de Ubajara, Rio das Minas, , el. 420 m, 20–23.iv.2012, DM Takiya, JA Rafael, F Limeira-de-Oliveira et al. cols., Malaise trap, male (CZMA).
Paratypes. Same data as holotype, 1 male (CZMA); same data, except 13–17.ix.2012, 25 males (CZMA), 12 males (DZRJ); same data, except 18–30.xi.2012, 9 males (INPA); same data, except 14–16.ii.2013, 5 males (MZUFBA); same data, except Rio das Minas, , el. 524 m, 14–16.ii.2013, DM Takiya, JA Rafael, RR Cavichioli & APM Santos cols., Malaise trap, 5 males (MNRJ).
Etymology. This species is named in reference to Ubajara National Park, in the municipality with the same name, where the types were collected from.
Remarks. This new species appears to be a member of the nigritta group because of their internal pouches between abdominal segments V and VI and the presence of two long subapical spines on phallus. Metrichia ubajara sp. nov. resembles M. potosina and M. goiana sp. nov. because of the rounded and elongate inferior appendages in lateral view. However, in M. ubajara sp. nov. the inferior appendages lack the “tooth” mentioned for these two species. As well, M. ubajara sp. nov. can be recognized by the phallic apex with a broad sclerotized plate wrapping the ejaculatory duct.
Metrichia vulgaris sp. nov.
urn:lsid:zoobank.org:act:BBC0387F-C111-4CA7-834A-F3711F7707F7
(Fig. 25)
Figure 25: Metrichia vulgaris sp. nov., male genitalia.
(A) ventral view; (B) dorsal view; (C) lateral view; (D) phallus, dorsal view.Figure 26: Abdominal modifications of Metrichia species.
(A) M. acuminata sp. nov., segments VI and VII, dorsal view; (B) M. itabaiana sp. nov., segments V, VI, and VII, dorsal view; (C) M. longissima sp. nov., segments V, VI, and VII, dorsal view; (D) M. peluda sp. nov., segments V, VI, and VII, dorsal view; (E) M. simples sp. nov., segments VI and VII, lateral view.Adult male. Length 2.7–3.1 mm (n = 12). General color, in alcohol, brown. Head with no modifications. Ocelli 3. Antenna simple, 21-articulated. Maxillary palpus 5-articulated; labial palpus 3-articulated. Mesoscutellum with transverse suture. Metascutellum subtriangular. Anterior femur without processes. Tibial spur formula 1-3-4. Wing venation reduced in both wings. Abdominal segment VI with pair of internal pouches in posterodorsal area. Ventromesal process on segment VII present. Segment VIII shorter ventrally than dorsally. Male genitalia. Segment IX reduced dorsally; sternum subquadrangular (Fig. 25A); in lateral view, narrower anteriorly than posteriorly (Fig. 25C). Inferior appendage covered by long setae, apex excavated, posterodorsal margin acute and sclerotized; subtrapezoidal in ventral view (Fig. 25A); in lateral view, subretangular (Fig. 25C). Dorsal hook long and stout, almost reaching the inferior appendage apex; in lateral view, downturned (Fig. 25C). Preanal appendage elongate, rounded and bearing very long setae (Fig. 25B). Subgenital plate apparently absent. Tergum X membranous and rounded (Fig. 25B). Phallus tubular, elongate and slender, slightly constricted mesally; with two short subapical spines; apex rounded and folded; ejaculatory duct sclerotized and protruding apically (Fig. 25D).
Holotype. BRAZIL: Rio de Janeiro: Itatiaia, Rio Palmital, , el. 637 m, 07.iii.2008, LL Dumas, JL Nessimian & MR de Souza cols., light trap, male (DZRJ).
Paratypes. Same data as holotype, 1 male (DZRJ), 1 male (MNRJ); same data, except Rio das Pedras, , el. 706 m, 06.iii.2008, LL Dumas, JL Nessimian & MR de Souza cols., light trap, 4 males (MNRJ). Ceará: Ubajara, Parque Nacional de Ubajara, Rio Gameleira, , el. 874 m, 20–22.iv.2012, F Limeira-de-Oliveira et al. cols., Malaise trap, 1 male (CZMA). Goiás: Alto Paraíso, Rio Bartolomeu tributary, , el. 1,270 m, 22–25.iii.2013, APM Santos & DM Takiya cols., Malaise trap, 3 males (DZRJ).
Etymology. This new species is named in allusion to its unusually wide distribution throughout Brazil. From the Latin “vulgaris” meaning “common.”
Remarks. This new species belongs to the campana group due to their internal pouches between segments VI and VII and the two small subapical spines in phallus. Metrichia vulgaris sp. nov. shares with M. campana, M. similis, and M. itabaiana sp. nov. the general aspect of inferior appendages, with an excavated posterior margin. From these species, M. vulgaris sp. nov. can be distinguished by their inferior appendages, in lateral view, excavated but with ventral corner more rounded than acute and short and stout dorsal hook and tergum X short and rounded.
Metrichia vulgaris sp. nov. has an interesting distributional pattern, occurring in very distant localities in Southeastern, Centralwestern, and Northeastern Brazil. In addition to their geographic distance, these localities are included in very distinctive areas: encompassing three biomes (Atlantic Forest, Cerrado, and Caatinga) and four large river basins (East North Atlantic, Southeast Atlantic, São Francisco, and Araguaia-Tocantins). Barcode sequences corroborate these different populations as the same species, with K2P intraspecific divergences up to 4.8%. This is the highest intraspecific divergence found in our work, but this value is still lower than that observed among species in other caddisfly groups (Pauls et al., 2010; Zhou et al., 2011). GMYC analyses grouped sequences into two ‘species,’ but these groups were not related to their geographic distribution: one group included samples from Rio de Janeiro and Minas Gerais and the other group, samples from Rio de Janeiro, Minas Gerais, and Goiás. The broader geographical and specimen sampling of this species, which is directly associated with its higher intraspecific divergences, may explain the oversplitting by the GMYC method, as discussed by Talavera, Dincă & Vila (2013).
Although such wide distribution is not common for Metrichia species, other microcaddisflies can show continental distributions (e.g., Oxyethira tica). Because the knowledge about Neotropical microcaddisflies is very poor, this pattern may be more common than currently thought.
Discussion
Although GMYC analysis overestimated the number of Metrichia species in our study (suggesting the split of M. circuliforme sp. nov. and M. vulgaris sp. nov. each into two ‘species’), COI sequences strongly corroborated species limits previously defined based on morphological features. In general, COI sequences of caddisflies show a robust ‘barcoding gap’ (Pauls et al., 2010; Zhou et al., 2011), making this molecular marker appropriate as a source of additional information to corroborate species delimitation or associations of different life stages.
Microcaddisflies are extremely diverse and poorly known, and when associated with the morphological data, the use of molecular information can result in a more robust taxonomy for this group. Although methods such ABGD and GMYC should not be used alone to determine ‘species,’ they are useful tools to identifying ‘potential species’ (Puillandre et al., 2012; Talavera, Dincă & Vila, 2013), especially in very diverse groups and/or with dubious morphology-based identification. The wide distribution of Metrichia vulgaris sp. nov. could indicate the existence of different cryptic species; however, morphology and barcode data agreed to define this group as a single species. Although GMYC overestimated the number of Metrichia species in our analysis, we consider this method an important tool for preliminary distinction when taxonomic information is poor.
Based on the presence of abdominal modifications, such as internal pouches, external sclerotized plates, and brushes of long setae, and features of male genitalia, six species groups have been proposed for Metrichia (Flint Jr, 1972; Bueno-Soria & Holzenthal, 2003). These abdominal modifications usually arise from segments V, VI, and VII, and, in general aspect, are very distinctive from each other (Fig. 26), possibly representing non-homologous structures. Nevertheless, most of the species described herein fit in these previously defined groups. Present analyses of COI sequences (Fig. 2 and Supplemental Information 4) recovered neither the monophyly of Metrichia nor any of the species groups tested (aberrans, campana, and nigritta groups). However, these relationships need to be analyzed further with better taxon and molecular marker sampling.
Herein, we have also used molecular data to associate larvae and adults of M. bonita sp. nov. Larvae remain unknown for most Neotropical species of Trichoptera, and they are even less known for microcaddisflies. Rearing immatures is very difficult and association based in co-occurrence with adults is not possible when several species of the same genus co-occur. In this way, DNA barcodes are a powerful tool, allowing the association and description of immature stages (Shan, Yang & Sun, 2004; Pauls et al., 2010; Ruiter, Boyle & Zhou, 2013). Barcode reference libraries for caddisflies are available for specimens from other regions (Zhou et al., 2009; Zhou et al., 2011; Ruiter, Boyle & Zhou, 2013) and represent an important source of information for taxonomic work as well as for ecological and evolutionary studies. We expect that molecular data will become increasingly common for Neotropical caddisflies as it facilitates the understanding of their diversity in this region.