New sources of Sym2A allele in the pea (Pisum sativum L.) carry the unique variant of candidate LysM-RLK gene LykX
- Published
- Accepted
- Received
- Academic Editor
- Yuriy Orlov
- Subject Areas
- Genetics, Molecular Biology, Plant Science
- Keywords
- Legumes, Pea, Rhizobia, Symbiotic specificity, cv. Afghanistan, Sym2, LykX, LysM-RLK
- Copyright
- © 2019 Sulima et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. New sources of Sym2A allele in the pea (Pisum sativum L.) carry the unique variant of candidate LysM-RLK gene LykX. PeerJ 7:e8070 https://doi.org/10.7717/peerj.8070
Abstract
At the onset of legume-rhizobial symbiosis, the mutual recognition of partners occurs based on a complicated interaction between signal molecules and receptors. Bacterial signal molecules named Nod factors (“nodulation factors”) are perceived by the plant LysM-containing receptor-like kinases (LysM-RLKs) that recognize details of its structure (i.e., unique substitutions), thus providing the conditions particular to symbiosis. In the garden pea (Pisum sativum L.), the allelic state of Sym2 gene has long been reported to regulate the symbiotic specificity: for infection to be successful, plants with the Sym2A allele (for “Sym2 Afghan”, as these genotypes originate mostly from Afghanistan) require an additional acetylation of the Nod factor which is irrelevant for genotypes with the Sym2E allele (for “Sym2 European”). Despite being described about 90 years ago, Sym2 has not yet been cloned, though phenotypic analysis suggests it probably encodes a receptor for the Nod factor. Recently, we described a novel pea gene LykX (PsLykX) from the LysM-RLK gene family that demonstrates a perfect correlation between its allelic state and the symbiotic specificity of the Sym2A-type. Here we report on a series of Middle-Eastern pea genotypes exhibiting the phenotype of narrow symbiotic specificity discovered in the VIR plant genetic resources gene bank (Saint-Petersburg, Russia). These genotypes are new sources of Sym2A, as has been confirmed by an allelism test with Sym2A pea cv. Afghanistan. Within these genotypes, LykX is present either in the allelic state characteristic for cv. Afghanistan, or in another, minor allelic state found in two genotypes from Tajikistan and Turkmenistan. Plants carrying the second allele demonstrate the same block of rhizobial infection as cv. Afghanistan when inoculated with an incompatible strain. Intriguingly, this “Tajik” allele of LykX differs from the “European” one by a single nucleotide polymorphism leading to an R75P change in the receptor part of the putative protein. Thus, our new data are in agreement with the hypothesis concerning the identity of LykX and the elusive Sym2 gene.
Introduction
The mutualistic symbiosis formed by legume plants (family Fabaceae) and nodule bacteria (a diverse group of bacteria collectively called rhizobia) is an example of a highly specific interaction of symbiotic partners. Communication between plant and bacteria relies on an exchange of signal molecules, a process known as “molecular cross-talk” (Oldroyd, 2001; Cooper, 2007; Zipfel & Oldroyd, 2017). The roots of a legume plant excrete flavonoid compounds attracting rhizobia, that, in turn, begin to produce the bacterial signal molecules called Nod factors (Nodulation factors) (Spaink, 1995; Schultze & Kondorosi, 1998; Perret, Staehelin & Broughton, 2000; Geurts & Bisseling, 2002). Nod factors are lipochitooligosaccharide molecules that have specific decorations characteristic for a particular species, or even strains, of rhizobia. Legume plants possess receptors that are able to bind Nod factors in very low concentrations and recognize the fine structure of the ligands, including the specific moieties (Bensmihen, De Billy & Gough, 2011; Delaux et al., 2015; Geurts, Xiao & Reinhold-Hurek, 2016; also reviewed in Gough et al., 2018), so that only a perfectly fitting strain of rhizobia is allowed to enter the root through the root hairs. The successful recognition of a Nod factor activates a signal transduction cascade leading to a change in gene expression in root epidermal cells (Larrainzar et al., 2015; Liu et al., 2019), formation of bacterial microcolony on a tip of the root hair and growth of so-called “infection thread”; a structure inside the root hair through which rhizobia are delivered to root cells that form a nodule primordium (Downie & Walker, 1999; Perret, Staehelin & Broughton, 2000). If plant recognizes that the Nod factor of a particular bacterial strain does not fit, the aforementioned processes do not occur.
The phenomenon of the specificity of legume-rhizobial symbiosis was noticed at least 90 years ago, when the Russian geneticist Govorov (1928), followed by Russian microbiologist Razumovskaya (1937), described pea (Pisum sativum L.) specimens originating from Afghanistan as incapable of forming nodules in European soils (Govorov, 1928; Razumovskaya, 1937). Further on, some specimens of pea originating from several Middle East countries were described as failing to nodulate when interacting with rhizobia strains that are common in Europe (Lie, 1971; Lie, 1978; Lie, Timmermans & Ladizinski, 1982; Lie & Timmermans, 1983). These specimens, however, established symbiosis with strains isolated from the soils of Turkey, Israel and some other countries. Later it was found that all that strains were characterized by a specific modification of the Nod factor molecule, namely the additional acetylation at the reducing end of the chitin backbone in the C-6 position caused by the acetyltransferase nodX (Davis, Evans & Johnston, 1988; Firmin et al., 1993). Subsequently, in the work of Ovtsyna et al. (1998) it was shown that the gene nodX can be functionally replaced by the gene nodZ encoding a fucosyltransferase that fucosylates the reducing end of Nod factor.
This unusual plant nodulation trait was called the “Afghan” phenotype, since the typical genotype of pea with such a phenotypic manifestation originates from Afghanistan (today this genotype is known as cv. Afghanistan, JI1357 and NGB2150) (Young & Matthews, 1982; Tsyganov et al., 1999). In contrast, the trait of broad specificity—the ability to be nodulated by strains from both Europe and Middle East—was named the “European” phenotype. From the plant side, the manifestation of the “Afghan” phenotype has been shown to be controlled by a single gene named Sym2, that was subsequently localized in the Ist linkage group of the pea genome (Kozik et al., 1995; Kozik et al., 1996b). Since rhizobia require the unique modification of the Nod factor molecule to successfully interact with the “Afghan” peas, it was assumed that Sym2 may encode a Nod factor receptor with two different allelic states: Sym2A (from “Afghan”, highly specific), and Sym2E, or Sym2C (from “European” or “cultivated”, less specific) (Kozik et al., 1996a; Geurts et al., 1997), although formal evidence of it is still lacking.
In 2003, genes encoding LysM-domain receptor-like kinases (LysM-RLKs) that probably perceive the Nod factor were described in the model legumes Lotus japonicus (Regel) Larsen (NFR1 and NFR5) and Medicago truncatula Gaertn. (LYK3) (Limpens et al., 2003; Madsen et al., 2003; Radutoiu et al., 2003). Interestingly, Limpens et al. (2003) found a cluster of LysM-RLK genes in the Sym2-orthologous region of the M. truncatula genome and described one of them (LYK3) as the best candidate for encoding the Nod factor receptor. In 2008, Zhukov and colleagues discovered two pea homologs of L. japonicus NFR1, namely Sym37 and K1, located in linkage group I, just where Sym2 is located (Zhukov et al., 2008). Unfortunately, neither Sym37 nor K1 demonstrated any sequence features that could distinguish “Afghan” lines from “European” ones, although products of these genes still may play a role in determination of Sym2A-type specificity as parts of the protein complexes. In our previous work (Sulima et al., 2017), a new pea gene named LykX (or PsLykX, for Pisum sativum LysM Kinase eXclusive) located in the same region of linkage group I was described. When analyzing the polymorphism of its first exon, a minor haplotype was found which is unique for lines with the “Afghan” phenotype. Thus, LykX has become a new candidate for the Sym2.
One of the drawbacks of many previous works aimed at finding the Sym2 gene has been the use of a limited number of “Afghan” lines with high genetic uniformity; for example, they all possessed the same alleles of the Sym37 and K1 genes. This may be due to the fact that these lines are close relatives, or even descendants of the same original genetic line. More than 30 years ago, the study of wild varieties of pea from Afghanistan, Iran, Tibet and Turkey revealed seven varieties with the “Afghan” specificity, but no molecular-genetic analysis had been performed (Kneen & LaRue, 1984). In the present work, the similar approach was employed: we attempted to discover new “Afghan” forms among 122 pea samples originating from different regions of the Middle East. Our aim was to expand the sample of available “Afghan” lines, to evaluate the polymorphism of the LykX gene, and to see if it is associated with particular preferences towards rhizobia.
Materials & Methods
Plant material
The search for pea lines demonstrating the “Afghan” phenotype was conducted on the basis of material obtained from the VIR plant genetic resources gene bank (N. I. Vavilov All-Russian Institute of Plant Genetic Resources (VIR), St. Petersburg, Russia), kindly provided by Prof. M. A. Vishnyakova, head of Department of genetic resources of leguminous crops, and E. V. Semenova, curator of the pea collection. The choice of material was determined by the geographical origin of samples (the Middle East). The whole sample contained 122 pea lines (see Table S1).
Additionally, a number of pea cultivars and genetic lines with the confirmed “Afghan” phenotype from the collection of the laboratory of genetics of plant-microbial interactions of the All-Russia Research Institute for Agricultural Microbiology (ARRIAM; St. Petersburg, Russia) were used as control (Table S2). The line 701 of Pisum fulvum Sibth. & Smith (Israel, Valley of the Cross) was kindly provided by Sc.D. O.E. Kosterin (Novosibirsk, Russia).
Plant growth conditions
During the summer season of 2011, plants were grown in vegetation houses in conditions corresponding to the region’s climate (St. Petersburg, Leningrad Oblast, Russia). When necessary for control, plants were grown in Vötsch Industrietechnik VB 1014 growth chambers (Balingen, Germany) under the following conditions:
-
day/night: 16/8 hours;
-
temperature: 21 °C;
-
relative air humidity: 75%; illumination: 38000 lux.
The seeds were pre-sterilized with concentrated H2SO4 for between 10 and 15 minutes and then washed five times with distilled water. Sterilized seeds were germinated in Petri dishes filled with sterile vermiculite for 3 days at 27 °C, then seedlings were planted in pots. For large-scale experiments in vegetation houses, sterilization and preliminary germination of seeds was omitted.
Plants were grown in the 2 l pots filled with sand. When sterility was necessary, pots together with sand were heated in a dry-heat cabinet for 2 h at 200 °C. Mineral nutrition (Table S3) was added to substrate simultaneously with planting seedlings, 150 ml of solution per pot. When the analysis of the symbiotic phenotype was required, combined nitrogen in the form of ammonium nitrate was not added to the nutrient solution.
Bacterial strains
In experiments involving analysis of nodulation, two contrast strains of Rhizobium leguminosarum bv. viciae were used:
Strain R. leguminosarum bv. viciae RCAM1026 (= CIAM1026), used in ARRIAM as a model strain (Afonin et al., 2017). This strain is unable to colonize a plant with the “Afghan” phenotype due to absence of the nodX gene (nodX− strain).
Strain R. leguminosarum bv. viciae A1, first isolated in the Leningrad Oblast from nodules of the “Afghan” pea line (Chetkova & Tikhonovich, 1986), thus possessing the nodX gene (nodX+ strain).
For the visualization of symbiotic phenotype, the gusA-marked derivative of RCAM1026 (RCAM1026 gusA) with constitutive expression of gusA gene was used (Kirienko et al., 2018).
Bacteria cultivation
The strains were cultivated on solid medium No. 79 (Table S4) in a thermostat at a temperature of 28 °C for three days. In the case of streptomycin-resistant strain RCAM1026, streptomycin (100 g/ml) was added to the medium; in the case of strain A1, no antibiotics were added. Plants were inoculated with a suspension of bacteria: the culture (“lawn”) from one Petri dish was resuspended in 1 l of sterile distilled water (final CFU/ml : not less than 106) and the resulting solution was added to the substrate while planting the seedlings, 100 ml per pot.
Plant DNA extraction
DNA was extracted from young leaves (top or second-from-top node). Extraction was conducted according to the previously described CTAB protocol (Rogers & Bendich, 1985; Sulima et al., 2017).
Sequencing of DNA fragments
To amplify the first exons of LykX, primers were used as described in Sulima et al. (2017). The PCRs were performed in 0.5 ml eppendorf-type microcentrifuge tubes on an iCycler (Bio-Rad, Hercules, CA, USA) or Dyad (Bio-Rad) thermocycler using the ScreenMix-HS kit (Evrogen, Moscow, Russia). The PCR cycling conditions were as follows: 95 °C (5 min), 35 × [95 °C (30 s), Tm (varying depending on primers) (30 s), 72 °C (1 min)], 72 °C (5 min). The PCR fragments were sequenced using the ABI Prism3500xL system (Applied Biosystems, Palo Alto, CA, USA) at the “Genomic Technologies, Proteomics, and Cell Biology” Core Center of the ARRIAM (St.Petersburg, Russia). The resulting sequences have been deposited into the NCBI database under accession numbers MN187362 –MN187364, MN200353 –MN200358.
Analysis of sequences was conducted by means of MEGA7 software (Kumar, Stecher & Tamura, 2016). A Minimum Evolution tree was built using the modified Nei-Gojobori (assumed transition/transversion bias = 2) method (Zhang, Rosenberg & Nei, 1998). A bootstrap test was performed with 500 bootstrap replications. Alignments were done in MEGA7 and EMBL-EBI Clustal Omega web service (https://www.ebi.ac.uk/Tools/msa/clustalo/).
Genotyping of F2 population
To analyze the F2 sample obtained from the cross between lines K-3821 and NGB2150 carrying different alleles of LykX, the CAPS (cleaved amplified polymorphic sequence) marker was designed using the dCAPS Finder 2.0 online service (Neff, Turk & Kalishman, 2002). The primers used were the same as for amplification of the 1st exon. The digestion of SNP (single nucleotide polymorphism) site was performed by the Hpy188III restriction enzyme (New England Biolabs, Massachusetts, USA).
Microscopy
For detailed phenotype analysis, plants were grown in sand, as described above, while inoculated with suspension of RCAM1026 gusA culture. Four weeks after inoculation, 5 segments of roots 2–2.5 cm long were collected from each plant, leaving approximately two cm from the base of the root and two cm from the tips (if possible). Root segments were stained for 18 h in a solution of 50 mM Na-phosphate buffer (pH 7.0) containing 0.1% Triton, 5 mM K3Fe(CN6) (red blood salt), 5 mM K4Fe(CN6) (yellow blood salt) and 0.02 % 5-bromo-4-chloro-3-indolyl-bD-glucuronic acid (X-glc). For storage, stained segments were fixated with 4% paraformaldehyde in a TBS buffer (50 mM TrisHCl, 150mM NaCl, pH 7.5) with the addition of 0.1% Tween-20 and 0.1% Triton X-100 under a vacuum (7 min, 3–4 times) using a VacuuBrand ME 1C vacuum pump (Vacuubrand, Wertheim, Germany) and then dehydrated in graded ethanol series (from 10% to 70%). For observation, fixated samples were treated with the same ethanol series in reverted order and then with TBS buffer. Microscopic analysis was conducted using Zeiss Axiovert 35 inverted phase contrast microscope (ZEISS, Oberkochen, Germany) by 100-fold magnification. The photos were taken on the Canon EOS 4000D camera (Canon, Tokyo, Japan).
Results
Selection of pea lines with narrow symbiotic specificity
To increase the amount of the available “Afghan” lines, a sample of 122 pea lines originating from various regions of the Middle East (Table S1) was obtained from the VIR plant genetic resources gene bank. In the summer season of 2011, the nodulation phenotype of the lines was tested by inoculation with nodX+ or nodX− strains. Five lines were discovered which did not form nodules when inoculated with nodX− strain RCAM1026, but demonstrated normal nodulation with strain A1 (i.e., the typical “Afghan” phenotype; so did plants of cv. Afghanistan (NGB2150) as well) (Table 1). From among the other lines analyzed, there were several with decreased or increased numbers of nodules formed by either the RCAM1026 (nodX−) strain or A1 (nodX+) strain, but these were not included in subsequent analysis.
Allelism test between newly discovered and previously known Sym2A-lines
In order to determine whether the symbiotic phenotype of selected pea accessions was caused by the same gene as in the previously known “Afghan” lines (i.e., the Sym2 gene), an allelism test was conducted. F1 seeds obtained from the cross of previously reported “Afghan” lines NGB2150, K-6883 and K-6047-2 with lines selected from VIR gene bank, along with seeds of parental lines, were planted in pots with sterile sand and inoculated with the nodX− rhizobia strain RCAM1026 (see Materials and Methods). Additionally, two seeds of cv. Finale (characterized by the broad “European” symbiotic specificity and thus able to interact with the nodX− strain RCAM1026) were planted in each pot to confirm the success of the inoculation.
| VIR accession number | Nodules formed with R. leguminosarum RCAM1026 (nodX−) | Nodules formed with R. leguminosarum A1 (nodX+), m ± SD | Place of origin |
|---|---|---|---|
| K-1878 | 0 | 58.0 ± 5.8 | Afghanistan |
| K-3374 | 0 | 61.6 ± 7.9 | Turkmenistan |
| K-3821 | 0 | 65.6 ± 4.9 | Tajikistan |
| K-4902 | 0 | 50.8 ± 3.5 | Uzbekistan |
| K-6559 | 0 | 75.4 ± 8.9 | Afghanistan |
| K-6566 | 0 | 94.2 ± 6.2 | Afghanistan |
After 4 weeks of vegetation, F1 hybrids, like the plants of parental lines, did not form nodules with the nodX− strain RCAM1026. Plants of cv. Finale in the same pots formed, on average, about 40 nodules per plant (see Table S5). The lack of nodules in F1 plants indicates the allelism of genetic determinants of symbiotic phenotype in newly-described and previously known “Afghan” pea lines. Thus, new sources of Sym2A alleles have been identified: the lines K-1878, K-3374, K-3821, K-4902, and K-6559.
Sequencing of the first exon of LykX in the selected lines of pea
In our previous work (Sulima et al., 2017), a new LysM-RLK gene LykX (PsLykX) was identified as a promising candidate for the elusive gene Sym2, as it demonstrated the unique allelic state found only in two pea lines with the “Afghan” phenotype which leads to substitution of three amino acids in its corresponding protein: Q44R, N45Y, and A76D, all in the first LysM module of the receptor part. Here we sequenced the first exon of LykX (corresponding to the receptor domain of the putative protein) in the newly selected “Afghan” lines from the VIR gene bank, several lines with “European” phenotype from our laboratory collection, cv. Iran characterized by the leaky temperature-dependent “Afghan” phenotype (Lie, 1971; Kozik et al., 1996a), and a wild relative of garden pea, Pisum fulvum.
It was revealed that in the majority of lines with “Afghan” phenotype the 1st exon of LykX has the same allelic state that was discovered previously (Sulima et al., 2017). In lines K-3821 and K-3374, however, LykX demonstrated another allelic state with unique substitutions observed neither in “European” nor in other “Afghan” lines. These lines were called “Tajik”, according to the place of origin of line K-3821, which was identified first. Taken this new find into account, we suspected that the results of our initial allelism test might be ambiguous, since the similar non-nodulation phenotype in F1 would be observed if the block of nodulation in the “Tajik” lines is caused by genetically dominant determinant independent of Sym2. In order to test that new hypothesis, the nodulation trait in F2 progeny derived from the cross between “Tajik” line K-3821 and the typical “Afghan” line NGB2150 was analyzed in the inoculation experiment similar to the one described above. Of 119 F2 plants, none demonstrated normal nodulation with RCAM1026 strain regardless of the allelic state of LykX (homozygous “Afghan” allele, homozygous “Tajik” allele, heterozygote), while control plants of cv. Finale formed, on average, 65.3 ± 20 (m ±SD, n = 28) nodules per plant (see Table S6 and Fig. S1). This suggests that our initial interpretation of allelism test results was correct.
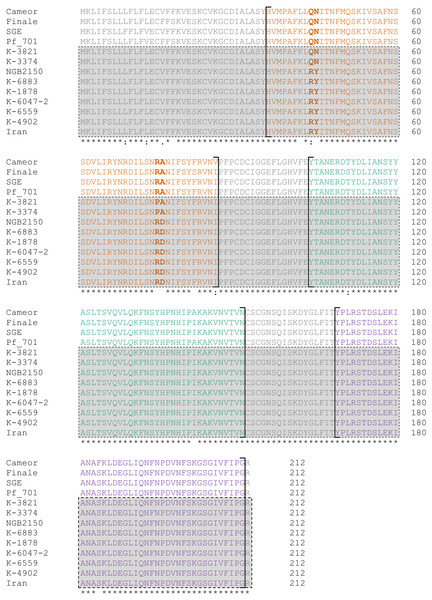
The nucleotide substitutions unique for “Afghan” lines lead to the replacement of three amino acids in the protein in comparison to the “European” lines (Table 2). “Tajik” lines seem to be much more similar to the “European” ones, with only one unique amino acid substitution in the protein: R75P. It is important to note that all these characteristic substitutions are concentrated in the first LysM module (LysM1) of the receptor domain, which could indirectly indicate the importance of this particular module for the functioning of the LykX protein (Fig. 1).
| Pea line | Substitution position in protein | |||
|---|---|---|---|---|
| 44 | 45 | 75 | 76 | |
| NGB2150 | R | Y | R | D |
| K-6883 | R | Y | R | D |
| K-6047-2 | R | Y | R | D |
| cv. Iran | R | Y | R | D |
| K-1878 | R | Y | R | D |
| K-4902 | R | Y | R | D |
| K-6559 | R | Y | R | D |
| K-3374 | Q | N | P | A |
| K-3821 | Q | N | P | A |
| Caméor | Q | N | R | A |
| Finale | Q | N | R | A |
| SGE | Q | N | R | A |
| P.fulvum 701 | Q | N | R | A |
Figure 1: Alignment of the putative receptor domain sequences of LykX protein from lines represented in Table 2.
LysM modules are highlighted by color (orange, LysM1; green, LysM2; violet, LysM3). Sequences corresponding to the lines with the Sym2A-type symbiotic specificity are boxed. Sites that distinguish “Afghan”, “European” and “Tajik” variants of LykX are given in bold.The plants of cv. Iran were found to carry the same allele of LykX as the cv. Afghanistan (NGB2150), which corresponds with the idea that their phenotype is determined by the same gene (Kozik et al., 1995; Kozik et al., 1996a). Interestingly, the “European” allele of LykX was found in Pisum fulvum line 701, the wild species closely related to Pisum sativum.
Phenotype confirmation
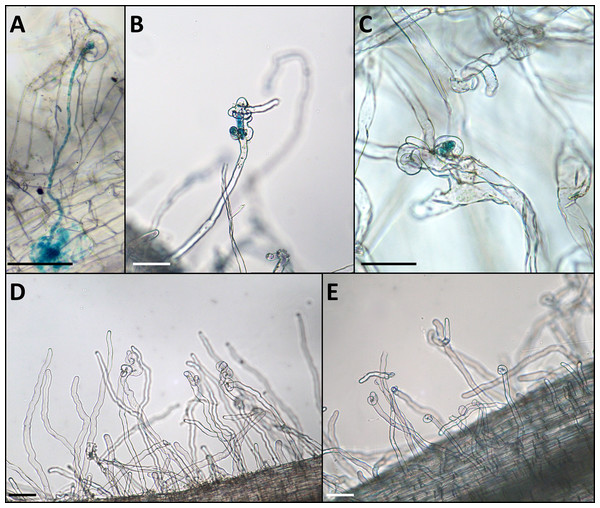
According to previous descriptions (Geurts et al., 1997; Zhukov et al., 2008), “Afghan” peas are unable to form normally developed infection threads while inoculated with the nodX− strain of rhizobia. Bearing this in mind, we performed the microscopic analysis of roots of “Tajik” lines in order to characterize their symbiotic phenotype. Seedlings of the “Tajik” line K-3821 and the “Afghan” line NGB2150, along with the “European” cv. Caméor, were inoculated with gusA-marked strain CIAM1026. 4 weeks after inoculation, the root fragments were harvested, treated with staining buffer and observed under a light microscope. Both lines demonstrated normal root hair curling and formation of microcolonies, but infection was aborted at this stage so no infection threads were observed for neither “Afghan” nor “Tajik” lines on 25 cm of roots (Figs. 2B, 2C). Control plants of cv. Caméor developed normal infection threads (Fig. 2A). Accordingly, plants of cv. Caméor formed 81.6 ± 15.2 (m ±SD, n = 10) nodules, while NGB2150 and K-3821 formed 0.2 ± 0.6 and 0.6 ± 1.3 nodules, respectively. Thus, we confirmed that “Afghan” and “Tajik” pea lines have an identical symbiotic phenotype, which is consistent with the alterations in the first exon sequences of LykX gene as compared to the “European” variant.
Figure 2: Phenotype of the infection threads in plants with different LykX alleles in the presence of nodX– strain RCAM1026 gusA.
(A) normal infection thread formed by the “European” line Caméor. (B, C) early abortion of the infection thread in “Afghan” line NGB2150 (B) and “Tajik” line K-3821 (C). (D, E) multiple curled root hairs of “Aghan” (D) and “Tajik” (E) plants. In most cases, the infection does not go beyond this stage. Scale bares are 0.1 mm.Discussion
Legume-rhizobial symbiosis relies on proper mutual recognition of partners, a process involving signal molecules and receptors that have been evolving step-by-step in a fashion similar to an “arms race”. Indeed, a plant needs to limit the set of bacteria penetrating its tissues as part of its defenses from pathogens and/or incompatible symbionts. On the other hand, bacteria may benefit when they broaden the range of possible hosts. Consequently, plants have evolved highly specific receptors that allow for discrimination of compatible and incompatible bacteria; in turn, rhizobia develop more and more variants of signal molecules (Price et al., 1992) that may be recognized by highly discriminatory plant receptors.
The pea is a unique object for studying the specificity of plant-microbe interactions due to the existence of a distinct group of specimens from Afghanistan showing a particular receptivity towards the microsymbiont. Such a phenotype is characteristic for plants carrying the Sym2A allele conferring “resistance” to nodulation with rhizobia strains producing the Nod factor without the required decoration. According to genotyping data (Jing et al., 2010), Afghanistan peas represent a distinct clade remote from both modern garden pea varieties and Pisum fulvum, the wild relative to Pisum sativum. Consistent with this, the recently-discovered LysM-RLK gene LykX is present among peas from Afghanistan and adjacent countries in a unique form that encodes a protein differing by three amino acid residues from that of “European” peas and of Pisum fulvum (Table 2). This unique haplotype is associated with a specific “Afghan” nodulation phenotype, which makes LykX a good candidate for the Sym2 gene. The “Afghan” haplotype of LykX was found in 3 previously uncharacterized lines originating from Afghanistan and Uzbekistan.
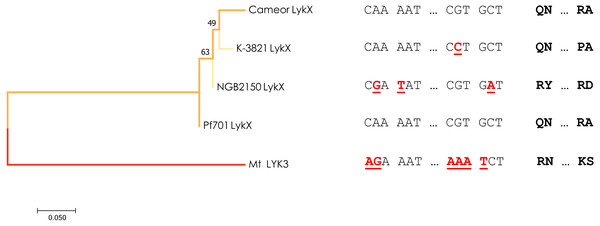
Interestingly, a different allele of LykX was discovered in two lines from Tajikistan and Turkmenistan manifesting the same strict symbiotic phenotype. To date we can’t confirm that a single nucleotide swap resulting in R75P amino acid substitution in the “Tajik” variant of LykX protein actually affects its functionality in a way that leads to a narrow receptivity, but proline is known by its increased conformational rigidity which indeed may have a significant effect on the secondary structure of the protein (Biedermannova et al., 2008). Yet, in the light of our recent findings, we can speculate that especially “choosy” forms of symbiosis may sometimes be an adaptive trait, as the “Tajik” allele of LykX arose from the “European” variant independently and more recently than the “Afghan” one, which is supported by a minimum-evolution tree constructed using the modified Nei-Gojobori method (Fig. 3). However, the reasons why peas growing in this particular region (the Middle East) had to narrow down their symbiotic specificity several times remain unclear. It can be assumed that certain environmental conditions (including the behavior of the native soil microbiota) forced them to more strictly limit the range of bacteria that have access to their root tissue.
Figure 3: Estimated evolutionary relationships between members of IRL clade of legumes based on sequence of the part of LykX gene or its orthologue corresponding to LysM1 module.
The evolutionary history was inferred using the Minimum Evolution method. The optimal tree with the sum of branch length = 0.640 is shown. On the right are the SNPs that distinguish the alleles and the amino acids corresponding to them. Pf701 : the LysM1 module of LykX gene in Pisum fulvum line 701. Mt LYK3 : the LysM1 module of LYK3 gene in Medicago truncatula Gaertn., the close orthologue of LykX.Pisum fulvum, which we used as an outgroup in the study, possesses the “European” variant of LykX, implying that it might form nodules with nodX− strains of Rhizobium leguminosarum. However, P. fulvum has been previously reported to form ineffective nodules with European strains (Lie, 1981), so it is likely that the infection stage of P. fulvum with nodX− strains may proceed normally. Another noteworthy pea accession in this work is cv. Iran, which, according to previous data, possesses the Sym2A allele (Kozik et al., 1995) and, as shown in current work, carries the “Afghan” allele of LykX. In our experiments it did not nodulate with the nodX− strain, although previously it has been documented that cv. Iran can form nodules with nodX− strains under high temperatures (28 °C) (Lie, 1971; Kozik et al., 1996a). Using Sym2A introgression lines, Kozik and colleagues have shown that temperature-dependent phenotype is intrinsic to cv. Afghanistan (NGB2150) as well, but is somewhat masked by the native genetic background (Kozik et al., 1996a). Our findings so far are in concert with these data, but the phenomenon in general may require further investigation. Moreover, there might be a chance of finding lines similar to cv. Iran in our sample of 122 pea accessions, as there are some lines with decreased numbers of nodules, and the temperatures in the Leningrad Oblast reached up to 30 °C during the summer of 2011.
Thus, our data are in agreement with the hypothesis of the identity of Sym2 and LykX. Accordingly, it seems appropriate to name the newly discovered allele of LykX Sym2T (for “Tajik”). However, genetic transformation experiments are still necessary to conclusively confirm or reject this hypothesis. It would also be essential to study the interaction of “Tajik” lines with rhizobial strains producing fucosylated Nod factor due to the presence of the nodZ gene, as their range of specificity may differ from that of the “typical Afghan” lines.
Conclusions
In this work we identified a series of naturally-occurred forms of pea (Pisum sativum L.) with the narrow symbiotic specificity similar to that of cv. Afghanistan, and confirmed that their phenotype is prompted by the same genetic determinant Sym2. It is highly likely that the recently discovered gene LykX is in fact the elusive Sym2 gene. Indeed, we found that within our sample, LykX demonstrates two allelic states (“Tajik” and “Afghan”) different from those found in forms with normal symbiotic receptivity (“European”). Both allelic states lead to different amino acid substitutions in their corresponding proteins, as compared to the more common “European” state. In neither K1 nor Sym37 –the previous candidate genes for Sym2 – were observed such correlations between allelic states and the “Afghan” phenotype. The results of our work also highlight the importance of the strict specificity of symbiosis for the pea, since, according to our data, it appeared independently at least twice during the evolution of Pisum sativum species.
Supplemental Information
The results of digestion of 1st exon of LykX with Hpy188III restrictase
Part of the the F 2 (K-3821 x NGB2150) sample is shown. Parental lines are marked as 3821 and 2150.
Pea specimens obtained from the VIR plant genetic resources gene bank
Assessment of nodule formation has been performed qualitatively: 0 = “–”; <10 = “–/+”; 10-30 = “+/–”; 40–60 = “+”; 70–90 = “++”; >100 = “+++”. For all lines, the number of plants per pot has been 5. *: In (Zhukov et al., 2008), the line K-1887 (VIR1887) with the narrow symbiotic specificity from the ARRIAM collection has been mentioned. There has been probably a mistake either in ARRIAM or in VIR protocols, and these two lines are the same. Here, we keep the numeration of VIR collection. **: Line K-6566 has been lost during the work.
The protocol for the preparation of the mineral nutrition solution for plants (according to Borisov et al., 1997, with modifications)
Concentrations are given for 1×working solution. Stock solutions are prepared separately for (1) MgSO 4 * 7H 2O, (2) K 2HPO 4, Ca 3(PO 4) 2, (3) NH 4NO 3, (4) NaFe-EDTA and (5) microelements, and then diluted to the required concentration. Stock solutions for (1), (2), (3), (4) are 100×. Stock solution for (5) is 1,000×. NH 4NO 3 is not to be added to the solution when analyzing the nodulation capability.
The results of the allelism test between the previously known “Afghan” lines and specimens from the VIR collection
C1,2, control plants of cultivar Finale (“European” phenotype); M, mean; MC, mean for control plants.
The results of the analysis of F 2 derived from the cross between “Tajik” line K-3821 and the typical “Afghan” line NGB2150
The “allele” columns indicate the allelic state of LykX (A –“Afghan”, T –“Tajik”, H –heterozygote). The “nn” columns indicate the number of nodules formed by nodX − strain RCAM1026. One seed of cv. Finale was planted in each pot with four F 2 plants to ensure successful nodulation; number of nodules formed by cv. Finale is listed in “cv.Finale” column. The alleles frequency corresponds to 1:2:1 for monogenic segregation (chi-square = 0.025 (P = 0.99)).