A molecular phylogeny of Geotrochus and Trochomorpha species (Gastropoda: Trochomorphidae) in Sabah, Malaysia reveals convergent evolution of shell morphology driven by environmental influences
- Published
- Accepted
- Received
- Academic Editor
- Edward Braun
- Subject Areas
- Evolutionary Studies, Molecular Biology, Taxonomy, Zoology
- Keywords
- Taxonomy, Borneo, Land snail, Shell sculpture, Shell size, Elevation, Precipitation, Phylogenetic signal, Mitochondrial gene, Nuclear gene
- Copyright
- © 2021 Chang and Liew
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. A molecular phylogeny of Geotrochus and Trochomorpha species (Gastropoda: Trochomorphidae) in Sabah, Malaysia reveals convergent evolution of shell morphology driven by environmental influences. PeerJ 9:e10526 https://doi.org/10.7717/peerj.10526
Abstract
There are currently eleven Geotrochus and four Trochomorpha species in Sabah. The primary diagnostic character that separates the two genera is the intensity of sculpture on the shell upper surface. All Trochomorpha species have a coarse nodular sculpture while Geotrochus species has a non-nodular sculpture or smooth shell. However, it is known that shell characters are often evolutionary labile with high plasticity in response to environmental factors. Hence, identifying the phylogenetic and ecological determinants for the shell characters will shed light on the shell-based taxonomy. This study aims to estimate the phylogenetic relationship between Geotrochus and Trochomorpha species in Sabah based in two mitochondrial genes (COI, 16S) and one nuclear gene (ITS) and also to examine the influence of temperature, elevation and annual precipitation on the coarseness of shell upper surface sculpture and shell sizes of the species of both genera. Additionally, we also investigated the phylogenetic signal of the shell characters. The phylogenetic analysis showed that Geotrochus and Trochomorpha species are not reciprocally monophyletic. The phylogenetic signal test suggested that shell size and upper surface sculpture are homoplastic, and these shell traits are strongly influenced by elevation and annual precipitation, particularly at the cloud zone of Mount Kinabalu. The highland species of both genera have a coarser shell surface than lowland species. The shell and aperture width decrease with increasing elevation and annual precipitation. In the view of finding above, the current taxonomy of Geotrochus and Trochmorpha in this region and elsewhere that based on shell characters need to be revised with sufficient specimens throughout the distribution range of the two genera.
Introduction
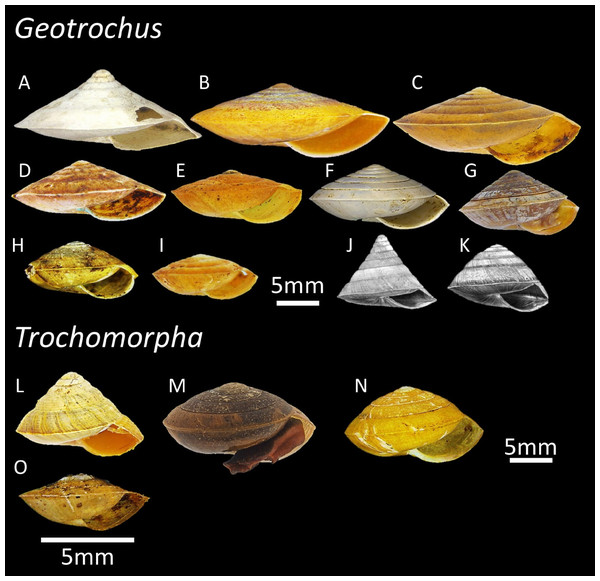
Geotrochus and Trochomorpha are two land snail genera that with similar shell forms belonging to the family Trochomorphidae (Fig. 1). The species of the two genera are ground-dwelling snails typically spotted on the understory vegetation and with overlapping distribution ranges in the region of Oceania and Southeast Asia (File S1). A recent revision of both genera reveals a total of eleven Geotrochus species and four Trochomorpha species in Sabah (Vermeulen, Liew & Schilthuizen, 2015). Trochomorpha species are endemic to montane forest and subalpine forest between 1,500 m and 3,400 m on Mount Kinabalu and Crocker Range in Sabah, while Geotrochus species are widespread in Sabah occur from lowland forest at sea level to highland until 2,400 m (Table 1; Vermeulen, Liew & Schilthuizen, 2015).
Figure 1: The variation of shell forms of 11 Geotrochus species and four Trochomorpha species in Sabah.
(A) G. conicoides (BOL/MOL 2431). (B) G. paraguensis (BOL/MOL 13061). (C) G. kinabaluensis (BOL/MOL 13020). (D) G. labuanensis (BOL/MOL 904). (E) G. oedobasis (BOL/MOL 908). (F) G. subscalaris (BOL/MOL 2430). (G) G. meristotrochus (BOL/MOL 13833). (H) G. whiteheadi (BOL/MOL 4110). (I) G. kitteli (BOL/MOL 4109). (J) G. spilokeiria (image from Vermeulen, Liew & Schilthuizen, 2015, CC BY 4.0). (K) G. scolops (image from Vermeulen, Liew & Schilthuizen, 2015, CC BY 4.0). (L) T. trachus (BOL/MOL 2959). (M) T. haptoderma (BOL/MOL 6312). (N) T. rhysa (BOL/MOL 3986). (O) T. thelecoryphe (BOL/MOL 6334).| Species | Specimens for phylogenetic analysisa | Quantitative shell traitsb | Upper shell sculpture typec | Elevational range | |||
|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | ||||
| Geotrochus conicoides (Metcalfe, 1851) | NA | NA | NA | NA | NA | NA | 63 m–363 m |
| Geotrochus kinabaluensis (Smith, 1895) | 2 | 4 | – | – | 3 | 1 | 16 m–2,001 m |
| Geotrochus kitteliVermeulen, Liew & Schilthuizen, 2015 | 1 | 2 | – | 4 | – | – | 1,563 m–2,376 m |
| Geotrochus labuanensis (Pfeiffer, 1863) | NA | 16 | – | – | 14 | 2 | 1 m–1,494 m |
| Geotrochus meristotrochusVermeulen, Liew & Schilthuizen, 2015 | 5 | 27 | – | – | 21 | 6 | 9 m–1,680 m |
| Geotrochus oedobasisVermeulen, Liew & Schilthuizen, 2015 | 3 | 6 | – | 1 | 5 | – | 260 m–2,291 m |
| Geotrochus paraguensis (Smith, 1893) | 8 | 10 | – | – | 9 | 1 | 1 m–756 m |
| Geotrochus scolopsVermeulen, Liew & Schilthuizen, 2015 | NA | NA | NA | NA | NA | NA | 718 m |
| Geotrochus spilokeiriaVermeulen, Liew & Schilthuizen, 2015 | NA | NA | NA | NA | NA | NA | 1,241 m |
| Geotrochus subscalarisVermeulen, Liew & Schilthuizen, 2015 | NA | 10 | – | – | 5 | 5 | 6 m–988 m |
| Geotrochus whiteheadi (Smith, 1895) | 1 | 1 | – | – | 1 | – | 827 m–2,080 m |
| Trochomorpha haptodermaVermeulen, Liew & Schilthuizen, 2015 | 8 | 7 | 43 | – | – | – | 2,055 m–3,360 m |
| Trochomorpha rhysaTillier & Bouchet, 1988 | 6 | 5 | 26 | – | – | – | 1,677 m–3,263 m |
| Trochomorpha thelecorypheVermeulen, Liew & Schilthuizen, 2015 | 1 | 0 | 8 | – | – | – | 1,990 m–2,992 m |
| Trochomorpha trachusVermeulen, Liew & Schilthuizen, 2015 | NA | NA | NA | NA | NA | NA | 1,563 m–1,815 m |
Notes:
- NA
-
No suitable shell was for the DNA data, shell quantitative traits measurement, or shell surface sculpture examination
- –
-
No specimen of the species belongs to the shell surface sculpture type
Taxonomy of Geotrochus and Trochomorpha in Sabah has been mainly based on shell and anatomical characters (Tillier & Bouchet, 1988; Vermeulen, Liew & Schilthuizen, 2015). Trochomorpha rhysa is the first species of Trochomorpha species described from Sabah (Tillier & Bouchet, 1988) from Mount Kinabalu between 3,000 m and 3,500 m. This new species was placed under Trochomorpha based on the genitalia and radula characters. After that, more new species of Trochomorpha and Geotrochus were described solely based on the shell characters (Vermeulen, Liew & Schilthuizen, 2015). Vermeulen, Liew & Schilthuizen (2015) noted that these species of the two genera have a similar shell, but Trochomorpha species have a coarser nodular sculpture on the upper surface of the shell.
Taxonomy of land snails based on anatomy and shell characters are not without its weakness because many of these characters are evolutionary labile (Pfenninger, Bahl & Streit, 1996; Liew, Schilthuizen & Vermeulen, 2009; Holznagel, Colgan & Lydeard, 2010; Hyman & Ponder, 2010; Hirano, Kameda & Chiba, 2014; Dowle et al., 2015; Köhler & Criscione, 2015). This open a question to what extent the shell upper surface sculpture is phylogenetically informative in Geotrochus and Trochomorpha as shell surface sculpture is known to evolve rapidly and in parallel or convergently in response to environmental conditions (Pfenninger & Magnin, 2001; Liew, Schilthuizen & Vermeulen, 2009). Therefore, it is vital to examine the phylogenetic relationship among Trochomorpha and Geotrochus species and the influences of habitat climatic factors to clarify the taxonomy of the two genera in Sabah as a way forward to improve the taxonomy of the two genera in Oceania and Southeast Asia in general.
Hence, this study aims to estimate the molecular phylogenetic relationship of selected species of Geotrochus and Trochomorpha species in Sabah by using two mitochondrial genes (COI and 16S) and one nuclear gene (ITS-1). After that, we examined the association of the shell size and shell upper surface sculptures with several environmental variables in their habitats. Lastly, the phylogenetic signal of the shell characters was tested.
Materials & Methods
Samples
All the eleven Geotrochus and four Trochomorpha species from Sabah are available in the BORNEENSIS Mollusca collection of Institute of Tropical Biology and Conservation in Universiti Malaysia Sabah. However, not all specimens of the species were suitable for phylogenetic and morphological analysis (Table 1). A total of six Geotrochus species, namely, G. meristotrochus (Vermeulen, Liew & Schilthuizen, 2015), G. kinabaluensis (Smith, 1895), G. paraguensis (Smith, 1893), G. oedobasis (Vermeulen, Liew & Schilthuizen, 2015), G. kitteli, (Vermeulen, Liew & Schilthuizen, 2015), and G. whiteheadi (Smith, 1895); and three Trochomorpha species, namely, T. haptoderma (Vermeulen, Liew & Schilthuizen, 2015), T. rhysa (Tillier & Bouchet, 1988), and T. thelecoryphe (Vermeulen, Liew & Schilthuizen, 2015) were available phylogenetic analysis. For morphological analysis, a total of 155 specimens of eight Geotrochus and three Trochomorpha species with intact shells were chosen to obtain quantitative and qualitative measurements. As there is no good quality specimen in the collection for Trochomorpha trachus (Vermeulen, Liew & Schilthuizen, 2015), Geotrochus conicoides (Metcalfe, 1851), Geotrochus spilokeiria (Vermeulen, Liew & Schilthuizen, 2015) and Geotrochus scolops (Vermeulen, Liew & Schilthuizen, 2015), these species were not included in the present study. Field sampling was approved by the Sabah Parks for Mt.Kinabalu, Tambuyukon, Mahua, Banggi Island and Balambangan Island, and Yayasan Sabah for INIKEA project site, Imbak Canyon and Maliau Basin (Permit: TTS/IP/100-6/2 Jld.7(70), 2018; Maliau Basin TTRP Project No. 228, 2017; and ICCA Expedition 2017).
| Collection reference numberaof the voucher specimens (BOR/MOL) | Taxon | Locationb | Sequencec | ||
|---|---|---|---|---|---|
| COI | 16S | ITS-1 | |||
| 6347 | Trochomorpha rhysa | Mount Kinabalu at 3,024 m | MK779474 | MK334188 | MK335437 |
| 6350 | Trochomorpha rhysa | Mount Kinabalu at 3,088 m | MK779475 | MK334190 | MK335439 |
| 6353 | Trochomorpha rhysa | Mount Kinabalu at 2,944 m | MK779477 | MK334191 | NA |
| 6354 | Trochomorpha rhysa | Mount Kinabalu at 2,944 m | MK779479 | NA | MK335440 |
| 6407 | Trochomorpha rhysa | Mount Kinabalu at 3,221 m | MK779478 | MK334195 | MK335444 |
| 6411 | Trochomorpha rhysa | Mount Kinabalu at 3,119 m | MK779476 | MK334196 | MK335446 |
| 6312 | Trochomorpha haptoderma | Mount Kinabalu at 2,775 m | NA | MK334185 | MK335433 |
| 6349 | Trochomorpha haptoderma | Mount Kinabalu at 2,896 m | MK779473 | MK334189 | MK335438 |
| 6356 | Trochomorpha haptoderma | Mount Kinabalu at 2,800 m | MK779472 | MK334192 | MK335441 |
| 6408 | Trochomorpha haptoderma | Mount Kinabalu at 2,484 m | MK779471 | NA | NA |
| 6409 | Trochomorpha haptoderma | Mount Kinabalu at 2,526 m | MK779470 | NA | MK335445 |
| 6412 | Trochomorpha haptoderma | Mount Kinabalu at 2,500 m | MK779469 | MK334197 | MK335447 |
| 6413 | Trochomorpha haptoderma | Mount Kinabalu at 2,404 m | MK779468 | NA | MK335448 |
| 6417 | Trochomorpha haptoderma | Mount Kinabalu at 2,896 m | MK779467 | NA | MK335449 |
| 6335 | Trochomorpha thelecoryphe | Mount Kinabalu at 2,700 m | MK779480 | NA | MK335434 |
| 6342 | Geotrochus oedobasis | Mount Kinabalu at 2,100 m | MK779461 | MK334186 | MK335435 |
| 6404 | Geotrochus oedobasis | Mount Kinabalu at 2,200 m | MK811549 | MK334193 | MK335442 |
| 6343 | Geotrochus oedobasis | Mount Tambuyukon at 2,080m | MK811548 | NA | NA |
| 6344 | Geotrochus whiteheadi | Mount Tambuyukon at 2,080 m | MK811544 | MK334187 | MK335436 |
| 6406 | Geotrochus kitteli | Mount Kinabalu at 2,300 m | MK779460 | MK334194 | MK335443 |
| 12670 | Geotrochus kinabaluensis | Crocker Range, Mahua at 1,200 m | MK811543 | NA | MK335450 |
| 13017 | Geotrochus kinabaluensis | Crocker Range, Mahua at 1,200 m | MK811542 | NA | NA |
| 13016 | Geotrochus meristotrochus | Tawau, INIKEA site at 200 m | MK811545 | MK334198 | MK335451 |
| 13323 | Geotrochus meristotrochus | Imbak Canyon Conservation Area between 400 and 600 m | MK811547 | MK334204 | MK335459 |
| 13325 | Geotrochus meristotrochus | Imbak Canyon Conservation Area between 400 and 600 m | MK811546 | MK334205 | MK335460 |
| 13373 | Geotrochus meristotrochus | Maliau Basin Conservation Area between 400 and 600 m | NA | NA | MK335461 |
| 13376 | Geotrochus meristotrochus | Maliau basin Conservation Area between 400 and 600 m | NA | NA | MK335462 |
| 13061 | Geotrochus paraguensis | Kudat, Banggi Island between 50–800 m | MK811550 | MK334199 | MK335452 |
| 13176 | Geotrochus paraguensis | Kudat, Banggi Island between 50–800 m | MK811552 | MK334200 | MK335454 |
| 13177 | Geotrochus paraguensis | Kudat, Banggi Island between 50–800 m | MK811551 | MK334201 | MK335455 |
| 13223 | Geotrochus paraguensis | Kudat, Banggi Island between 50 and 800 m | MK779464 | MK334202 | MK335456 |
| 13224 | Geotrochus paraguensis | Kudat, Banggi Island between 50–800 m | MK779465 | NA | MK335457 |
| 13225 | Geotrochus paraguensis | Kudat, Banggi Island between 50–800 m | MK779463 | MK334203 | MK335458 |
| 13068 | Geotrochus paraguensis | Kudat, Balambangan Island between 20–100 m | MK779462 | NA | MK335453 |
| 13084 | Geotrochus paraguensis | Kudat, Balambangan Island between 20–100 m | MK779466 | NA | NA |
Notes:
- NA
-
The DNA sequence was not available as the amplification of the gene was not successful.
DNA extraction, amplification and sequencing
Foot muscle with about two mm3 was excised from the preserved land snails using a sterilised scalpel. Genomic DNA was extracted using DNeasy Blood and Tissue Kit (Qiagen Inc., Hilden, Germany) following the standard procedure of the manual. Each of the two mitochondrial genes fragment was amplified by using primer pair LCO1490 and HCO2198 (Folmer et al., 1994) with an annealing temperature of 54 °C for COI; and primer pair 16Sbr-L and 16Sbr-H (Palumbi et al., 1991) with an annealing temperature of 47 °C for 16S. One nuclear gene fragment (ITS-1) were amplified using the primer pair 5.8c ‘silkworm’ and 18d’ fruitfly’ (Hillis & Dixon, 1991) with an annealing temperature of 55 °C. The PCR thermal-cycling profile includes initial denaturation at 94 °C for 3 min, followed by 35 cycles of denaturation at 94 °C for 30s, annealing at a locus-specific temperature for each primer for 45s, extension at 72 °C for 1 min and a final extension at 72 °C for 5 min. Positive PCR products were then sent to MyTACG Bioscience Enterprise for sequencing by using the forward and reverse primers that were used during PCR.
Sequence alignment and molecular phylogenetic reconstruction
The resulting forward and reverse sequences were assembled and aligned in Bioedit 7.2.6 (Hall, 1999), and the sequences were deposited in GenBank (Table 2). A total of four DNA sequence data matrix were made—one for each of the markers (16S, ITS, and COI) and one concatenated data matrix of the three markers. For the data matrixes with one marker, each was tested for molecular substitution model by using ModelFinder (Kalyaanamoorthy et al., 2017) based on the both AIC and BIC that built into IQ-Tree v.1.6.7 (Nguyen et al., 2015; Trifinopoulos et al., 2016). However, the COI data matrix was partitioned by codon positions before it was tested for the molecular substitution model.
For concatenated data matrix, it was partitioned by markers and codons (16S, ITS-1, first codon positions of COI, second codon positions of COI, and third codon positions of COI). Each of the partitions was tested for molecular evolution via ModelFinder (Kalyaanamoorthy et al., 2017) and partition models (Chernomor, Von Haeseler & Minh, 2016) based on the both AIC and BIC that built into IQ-Tree v.1.6.7 (Nguyen et al., 2015; Trifinopoulos et al., 2016). For all the analyses, we limited the candidate models to the six models that are available in MrBayes analysis, namely, JC, F81, K80, HKY, SYM and GTR. The phylogenetic analyses were performed based on the best partitioning scheme and substitution model for the respective markers and concatenated data matrix (File S2).
Next, we used Bayesian Inference (BI), and Maximum Likelihood (ML) approaches to reconstruct the phylogenetic trees by using MrBayes v3.2.6 (Huelsenbeck et al., 2001) and maximum likelihood (ML) method implemented in IQ-Tree v.2.1.1 (Nguyen et al., 2015) respectively for the concatenated data matrix and the data matrix for each of the three genes. All analyses were done in the CIPRES Science Gateway portal (Miller, Pfeiffer & Schwartz, 2010). The BI analysis was run for 1000000 generations along four chains with sample frequency set to 100 and a burn-in of 2500 (25%) (File S3). The phylogenetic trees generated from the two approaches were then viewed and edited using TreeGraph 2.14 (Stöver & Müller, 2010). Everettia klemmantanica (Dyakiidae) was selected as an outgroup because this species was the sister taxa of the Trochomorphidae (Bouchet et al., 2017).
Phylogenetic signal analysis
To investigate the influence of phylogeny on the evolution of shell upper surface sculpture and the four quantitative shell traits, the phylogenetic signal of these shell characters were assessed with Pagel’s Lambda (Pagel, 1999) and Blomberg’s K (Blomberg, Garland Jr & Ives, 2003). The analysis was performed by using “geiger” package (Harmon et al., 2008) and “phytol” package (Revell, 2012) in the environment of RStudio 1.1.4 (RStudio Team, 2015) following the method of Phung, Heng & Liew (2017) (File S4). We used the phylogenetic tree resulted from Maximum Likelihood (ML) but retained only one tip for each taxon, except for G. paraguensis which two tips were included—one for each of the two paraphyletic clades. For the qualitative shell trait, all the ten tips with nine species in the phylogenetic tree used for the phylogenetic analysis. However, for the quantitative shell traits, the tips of the phylogenetic tree represented by the juvenile specimen (i.e., T. thelecoryphe) were excluded (File S5).
Shell characters measurement
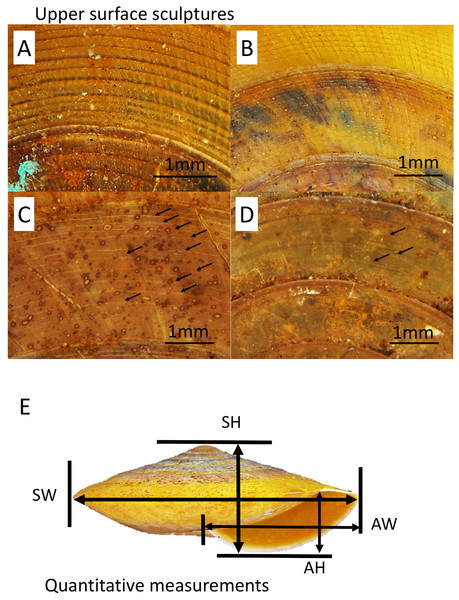
A total of five primary diagnostic shell characters that were used for delimitation of the species in Geotrochus and Trochomorpha were measured qualitatively and quantitatively (Fig. 2). The types of shell upper surface sculptures for the adult and subadult specimens with at least three whorls were recorded based on the four categories (S1–S4) of coarseness that are visible at 8× magnification. Sculpture S1—Densely placed, more or less regularly spaced radial riblets and between 11-19 spiral threads that form nodes over the radial sculpture; S2—Raised and distinct radial growth lines and 15 thin spiral threads; S3—Indistinct radial growth lines and inconspicuous riblets and between 6 and 23 thin or very thin spiral threads; and S4—Inconspicuous growth lines and between 4 and 25 low and thin spiral threads. There are a few species exhibit variability in the shell upper surface sculptures. Thus, the specimens of these species can be categorised into two shell upper surface sculpture types.
Figure 2: Upper surface sculptures and quantitative shell traits included in this study.
(A) Sculpture with spiral threads form nodes over radial sculpture (BOL/MOL 6312). (B) Sculpture with raised and distinct radial growth lines and thin spiral threads (BOL/MOL 6406). (C) Sculpture with indistinct radial growth lines and inconspicuous riblets and thin or very thin spiral threads (BOL/MOL 13061). (D) Sculpture with inconspicuous growth lines and low and thin spiral threads (BOL/MOL 890). (E) Four quantitative shell measurements: SH, Shell height; SW, Shell width; AH, Aperture height; and AW, Aperture width.Also, four quantitative measurements of shell size, namely, shell height (SH), shell width (SW), aperture height (AH) and aperture width (AW) were measured to nearest 0.1 mm from the photograph of the shell apertural view with the aid of Leica Stereo Microscope M205 (Fig. 2). Although there are other shell characters included in the description of each species by Vermeulen, Liew & Schilthuizen (2015), we only included these five primary diagnostic characters due to two reasons. First, the evolutionary and ecological aspects of these selected characters are better known since the review by Goodfriend (1986) and second, the other shell characters are species-specific.
Collection of ecological data
To investigate the correlation between shell size and upper surface sculpture and the environmental variables, we obtained the elevation, annual precipitation and temperature of the location where the specimens were collected. The elevation of the location was extracted from SRTM DEM 30-meter resolution (http://earthexplorer.usgs.gov/), and the annual precipitation and annual average temperature were extracted from global average temperature and annual precipitation layers of 30 arc-seconds (∼1 km) resolution of WorldClim v1.4 database (http://www.worldclim.org) using point sampling tool of QGIS v2.60 (QGIS Development Team, 2019). As expected, the annual average temperature is confounding with the elevation. Hence, we explored the influence of the elevation and annual precipitation to the shell sizes and shell surface sculptures, as suggested by Goodfriend (1986).
Statistical analysis
For some species, the specimens are relatively uniform in shell upper surface sculpture and belong to one of the four categories of sculpture intensity. In contrast, other species are variable in shell upper surface sculpture and belong to more than one category (Table 1). Hence, we treated a specimen as an observation unit (i.e., replicates) for each of the four categories regardless of the specimen’s species identity. We tested the null hypothesis (H0) of there is no difference in the elevation of the habitat among the between the snail with different shell upper surface sculpture intensity. Besides, we also tested the null hypothesis (H0) of there is no difference in the annual precipitation of the habitat among the between the snail with different shell upper surface sculpture intensity. As the data was not normally distributed, we performed Kruskal-Wallis tests to test the hypotheses (Kruskal & Wallis, 1952). Both analyses were performed in RStudio 1.1.4 (RStudio Team, 2015) (File S4).
We examined the collinearity of among the four shell size measurements. The results showed that aperture width (AW) is strongly correlated with shell width (SW) (r = 0.99), while the pairwise correlations among the other measurements are weaker with correlation coefficient values (r) range between 0.65 and 0.71. Hence, only SH, SW and AH measurements were retained for further analysis. All the three measurements were not normally distributed as reveal by Shapiro–Wilk test (Shapiro & Wilk, 1965). Therefore, Spearman’s correlation tests (Spearman, 1904) were employed to examine the relationships between each of the two environmental variables with the three shell measurements.
Results
Molecular phylogeny of Trochomorpha and Geotrochus species in Sabah
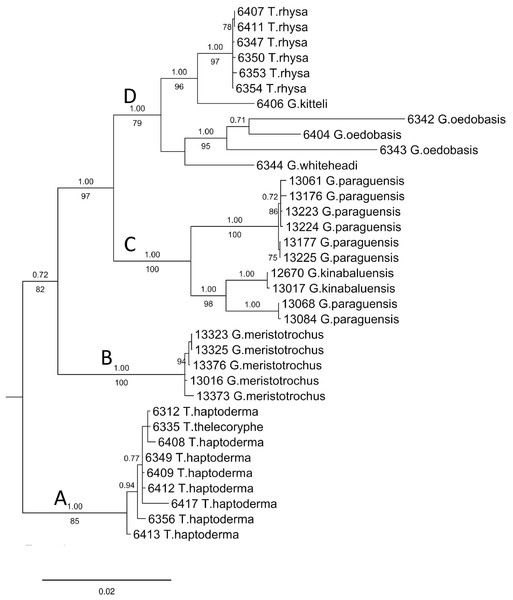
The final DNA alignment data matrix consists of 34 taxa and 1918 characters (16S: 1–461 bps; COI: 462–1,112; and ITS-1:1113–1918). The phylogenetic relationship of Geotrochus and Trochomorpha species of the concatenated dataset was shown in Fig. 3 (File S6). Generally, the phylogenetic trees estimated from each of the three genes show the topology as the tree estimated from the concatenated dataset (File S7). Generally, the three trees reconstructed based on the respective genes congruence to the tree that based combined genes, except for the taxa in Clade D. Particularly, G. oedobasis, G. kitteli, and G. whiteheadi that did not form a clade with T. rhysa in 16S and COI tree. On the other hand, all the taxa in Clade D appear to be polytomy in the ITS tree.
Figure 3: Bayesian inference tree of Geotrochus and Trochomorpha spp. based on concatenated dataset of 16S rDNA, COI and ITS-1 rooted to Everettia klemmantanica.
The letters A–D indicate the four major clades. Posterior probability (above the branch) from Bayesian inference and bootstrap support values (below the branch) from maximum likelihood analysis are indicated at the nodes with support values less than 0.7 of PP and 70% of BS were not shown in the figure. The number annotated in front of the species name was the BORNEENSIS collection number (Table 3).| Shell traits | Lambda (λ) | p-value | K | p-value |
|---|---|---|---|---|
| Upper surface sculpture | 0.000 | 1 | 1.021 | 0.067 |
| Maximum shell height | 0.638 | 0.565 | 0.954 | 0.108 |
| Maximum shell width | 1.000 | 0.258 | 0.994 | 0.070 |
| Maximum aperture height | 0.000 | 1 | 0.700 | 0.339 |
| Maximum aperture width | 0.855 | 0.456 | 0.895 | 0.124 |
For concatenated DNA data matrix, the analyses of ML and BI yielded a phylogenetic tree with an identical topology that with >79% bootstrap values for ML and 1.00 posterior probability values for the four major clades. Both ML and BI analyses showed that Geotrochus and Trochomorpha species are not monophyletic. Geotrochus kitteli is the sister taxon to Trochomorpha rhysa (Clade D), and T. thelecoryphe is nested in the T. haptoderma (Clade A). Geotrochus paraguensis from Banggi and Balambangan Island is paraphyletic with G. kinabaluensis (Clade C). Clade B contained G. meristotrochus.
Evidence for limited phylogenetic signal
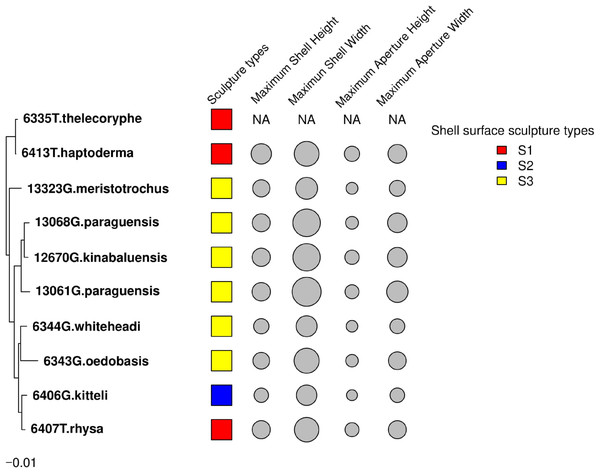
The results from these two approaches showed that the shell height, shell width, aperture height and aperture width of Geotrochus and Trochomorpha considered in this study did not show significant phylogenetic signal. Besides, the shell upper surface sculptures appear as homoplasy character (p > 0.05) (Fig. 4 and Table 3).
Figure 4: Shell upper surface sculpture types and quantitative shell’s traits were mapped on to the phylogenetic tree.
The shell upper surface sculpture types were represented by the different colour of the squares; and the four shell quantitative traits: maximum shell height, maximum shell width, maximum aperture height, maximum aperture width were represented by the size of the grey circle. The quantitative traits measurements were not available for T. thelecoryphe.Association between shell morphology and environmental variables
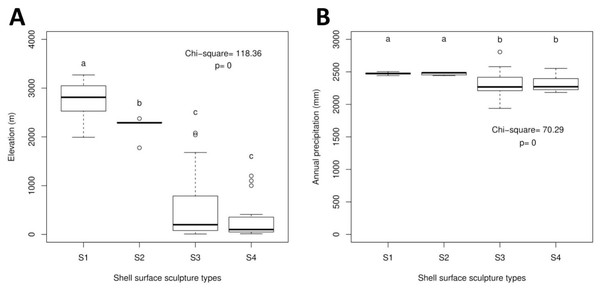
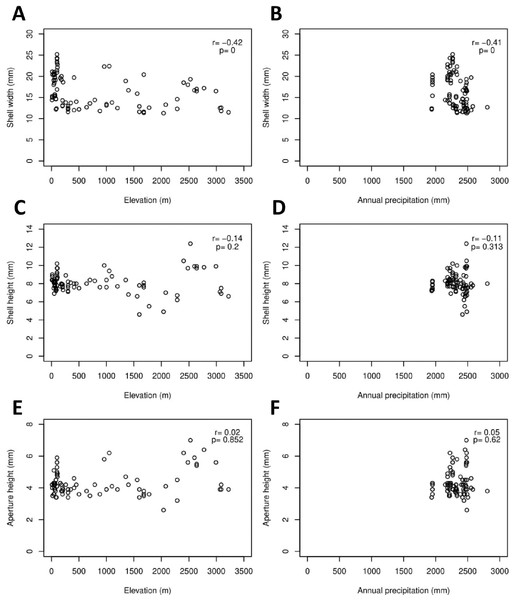
The Geotrochus and Trochomorpha species that have coarser shell surface sculpture (i.e., Type S1 and S2) tend to occupy habitats at higher elevation (above 2000 m) (Kruskal-Wallis X2 = 118.36, df = 3, p < 0.0001) and annual precipitation between 2400 mm and 2500 mm (Kruskal-Wallis X2 = 70.29, df = 3, p < 0.0001, Fig. 5, File S8). Shell width was negatively correlated with elevation (rs = − 0.42, p < 0.0001) and with annual precipitation (rs = − 0.41, p < 0.0001) (Fig. 6). On the other hand, shell height (rs = − 0.14, p > 0.2) and aperture height (rs = − 0.02, p > 0.9) were neither correlated with elevation nor annual precipitation (rs = − 0.11, p > 0.3; rs = − 0.05, p > 0.6).
Figure 5: Boxplots show the differences of the elevation and precipitation of the habitats of the shell with the four shell upper surface sculptures (S1–S4).
Kruskal-Wallis tests were performed, and the Chi-square values and the p-value of the test were shown in the plot. The alphabets above the boxplot indicate the results of multiple Wilcoxon signed-rank tests posthoc test. Sample sizes for each shell upper surface sculpture types were: S1 (n = 77); S2 (n = 5); S3 (n = 58); S4 (n = 15). (A) Differences of the elevation of the habitats of the shell with the four shell upper surface sculptures. (B) Differences of the annual precipitation of the habitats of the shell with the four shell upper surface sculptures.Figure 6: Correlations between shell quantitative traits (i.e., sizes) and environmental variables (elevation and precipitation).
Spearman correlation tests were performed, and the correlation coefficient values (r) and the p-value of the test were shown in the plot, n = 155. (A) A significant negative correlation between shell width and elevation. (B) A significant negative correlation between shell width and annual precipitation. (C) No significant correlation between shell height and elevation. (D) No significant correlation between shell height and annual precipitation. (E) No significant correlation between aperture height and elevation. (F) No significant correlation between aperture height and annual precipitation.Discussion
Phylogeny of Geotrochus and Trochomorpha and its implication to taxonomy
The phylogenetic analysis showed that Geotrochus and Trochomorpha are not reciprocally monophyletic (Fig. 3). This result is contrary to the current taxonomy of the two genera that was based on the shell characters, especially the shell upper surface sculpture. The confusing taxonomy of the two genera goes back to the description of Geotrochus by Van Hasselt (1823, but published in 1824) based on the specimens from Java Island, Indonesia, and the description of Trochomorpha by Albers (1850) based on several Geotrochus-like species from Southeast Asia and Pacific Islands. After that, Von Martens (1867) questioned the validity of the description of the genus Geotrochus by Van Hasselt (1823, published in 1824) as there is no type assigned to the genus. Hence, Von Martens (1867) concluded that the Geotrochus is morphologically similar to Trochomorpha, and he used Trochomorpha instead of Geotrochus as a valid genus for the land snails from Borneo. Later, Issel (1874) used only Trochomorpha for the species recorded in Borneo with no mention of Geotrochus at all. Until the year 1935, Pilsbry (1935) validated the genus Geotrochus based on the Opinions no. 46 rendered by the International Commission on Zoological Nomenclature. Solem (1964) used only Geotrochus for the checklist of land snails in Sabah.
The first detailed shell and anatomical description of the species of the two genera in Sabah were done by Tillier & Bouchet (1988) based on T. rhysa from Mount Kinabalu. Although the shell morphology, genitalia character and radula were described in detail, there was no comparison made to the known Geotrochus species or Trochomorpha species from other regions. In fact, Geotrochus was not mentioned at all in Tillier & Bouchet (1988). The first comprehensive revision on Geotrochus and Trochomorpha is by Vermeulen, Liew & Schilthuizen (2015) for the species in Sabah based on the shell morphology. There were four Trochomorpha species, of which three were new, and 11 Geotrochus, of which six were new were included in the revision (Vermeulen, Liew & Schilthuizen, 2015).
The taxonomy history of the two genera in Sabah that lead to their confusing taxonomy is not merely an isolated case but reflects the taxonomy problem of the two genera on a large scale. The two genera have been used interchangeably as seen in the records of the two genera in the museum worldwide (File S1). As revealed by the GBIF data, there is a large extent of the overlapping in the distribution ranges of the two genera. This pattern could represent a real situation or could be resulted from the misidentification of the species or genera given the fact that the shells of the species in the two genera are very similar. Schileyko (2002a) and Schileyko (2002b) recognised current taxonomy of Trochomorpha is still unresolved, and he placed Trochomorpha in the Family Trochomorphidae whereas Geotrochus in the Family Helicarionidae.
Our results indicate that more comprehensive taxonomy study on Trochomorpha and Geotrochus are needed, not only for the Sabah taxa but for the entire distribution ranges of the two genera. However, the fact that T. rhysa is more genetically closely related to the Geotrochus species implies that its putative taxonomy position was likely misled by the parallelism in genital character as documented occasionally occurred in other groups of land snails (e.g., Davison et al., 2005; Hirano, Kameda & Chiba, 2014). Moreover, the coarse nodular upper surface sculpture was also taxonomically uninformative as the shell character has found evolved independently in this study.
Regarding taxonomy at the species level, this study confirmed the existence of the eight genetically distinct species classified by Vermeulen, Liew & Schilthuizen (2015), except T. thelecoryphe and T. haptoderma. The two Trochomorpha species are very similar in the shell, but T. thelecoryphe has a flatter spire than T. haptoderma (Vermeulen, Liew & Schilthuizen, 2015). It is possible the type specimen of T. thelecoryphe in Vermeulen, Liew & Schilthuizen (2015) was a juvenile shell. Hence, more good condition specimens are needed for further clarification in a future study.
Evolution of shell surface sculpture coarseness and shell sizes of Geotrochus and Trochomorpha
Polyphyly of the genus Trochomorpha indicated that the diagnostic shell upper surface sculpture is a homoplasy character. Our results show that environments of the habitat influence the shell characters, and phylogenetic closely related species do not tend to resemble each other in the shell size and shell upper surface sculpture. Hence, these shell traits of Geotrochus and Trochomorpha are evolutionary labile that are not suitable to be served as diagnostic characters at the genus level.
The convergence of the shell traits is instead a common phenomenon among land snails that occupying similar ecological niches (Emberton, 1995; Phung, Heng & Liew, 2017). The physical shell is deemed to be the by-product of adaptation to their environmental attributes (Goodfriend, 1986; Baur & Raboud, 1988; Pfenninger et al., 2005; Proćków, Kuźnik-Kowalska & Mackiewicz, 2017; Proćków et al., 2018; but see Gittenberger, 1991; Fehér et al., 2018 for non-adaptive radiation). The rough surface of the shell helps land snail live with excessive water or moisture in their habitats. For example, ribbed shells retain more water on the shell surface (Giokas, Páll-Gergely & Mettouris, 2014); hairy shells increase the snails’ adherence wet surface of the plants in a more humid high-elevated area (Pfenninger et al., 2005; Proćków et al., 2018, but see Shvydka, Kovalev & Gorb, 2019); and coarser granular-like surface sculptures on shell help in reducing the water retention on the surface (Nosonovsky & Bhushan, 2008; Maeda et al., 2019). Besides, rough shells of other few ground-dwelling land snail species are known to be covered with soil that acts as camouflage (Páll-Gergely et al., 2015). From our field observation, we have not observed a shell of the living snail that is covered by soils or other materials. Hence, we suggest that the coarser shell surface helps Trochomorpha and Geotrochus species at highland elevation habitat dwell through fallen wet leaves by reducing the adhesiveness to its surrounding.
As can be seen from the records of the specimens and analysis (Table 1, Fig. 5), the snails with the coarser surface (i.e., S1 and S2) occur above 1500 m and more commonly above 2000 m. The abrupt transition of the shell surface is unlikely caused by temperature as the temperature generally decreases with increasing elevation (Whitmore, 1975; Kitayama, 1992; Kitayama, 1994). The occurrence of snails with a coarser shell upper surface (i.e., S1 and S2) at the area with relatively higher annual precipitation. Interestingly, these areas with relatively higher annual precipitation are also located at a higher elevation (>1,500 m).
In addition to rainfall, a substantial amount of precipitation may be added by horizontal rain in the cloud zone (Kitayama, 1992; Kitayama, 1994; Kitayama et al., 1998) or cloudy mossy forest (Frahm et al., 1990) between 2,000 m and 2,800 m. The habitat at this middle slope cloud zone with the increase in water surplus increased from 27% at 800m to 70% at 2,100 m (Kitayama, 1994; Kitayama et al., 1998). The species that predominantly with shell surface type S1 and S2 are endemic to Mount Kinabalu, namely, T. haptoderma, T. rhysa, T. thelecoryphe, and G. kitteli that are common above 2,000 m on the mountain.
The relationships between shell size and two significant environmental variables, namely, elevation and precipitation, are well documented (Goodfriend, 1986; Baur & Raboud, 1988; Pfenninger & Magnin, 2001; Glass & Darby, 2009; Anderson, Lew & Peterson, 2003; Proćków, Kuźnik-Kowalska & Mackiewicz, 2017). Our results show that the shell width and aperture width of the two genera are negatively correlated with elevation and annual precipitation. As the temperature is confounding with elevation, it also means that the shell size of the species in both genera follows converse Bergmann’s rule (Baur & Raboud, 1988; Anderson, Lew & Peterson, 2003; Proćków, Kuźnik-Kowalska & Mackiewicz, 2017). It was hypothesised that the colder environment induces highland land snail to reach sexual maturity faster than those living in the warmer area. Hence, shells of the highland land snails are often smaller as the growth of the land snails is limited after maturity (Proćków, Kuźnik-Kowalska & Mackiewicz, 2017).
It is known that there is a positive relationship between high precipitation and shell size of land snails because humid habitat promotes the growth and expansion rate of shell whorls (Goodfriend, 1986). However, this may not be the case for montane species (Goodfriend, 1986; Proćków, Kuźnik-Kowalska & Mackiewicz, 2017). Our results show that Geotrochus and Trochomorpha species from sites with lower annual precipitation have a larger shell size. Although there is a statistically significant difference in the annual precipitation, we suggest that the precipitation per se might not be the only factor as the species of S1 and S2 that occur above 1500 m on the Mount Kinabalu are also experiencing horizontal precipitation resulted from the Middle slope wet cloud zone on Mount Kinabalu.
The negative correlation could probably due to the favourable effect of moisture on shell size has been compensated by the lower temperature on the high elevation that generally has a negative effect on shell size (Goodfriend, 1986; Baur & Raboud, 1988; Anderson, Lew & Peterson, 2003). Besides, decreasing in aperture size with the altitudinal gradient has generally been interpreted as an adaptation to the lower humidity at the lower elevational area (Goodfriend, 1986) as smaller apertures tend to lose proportionately more water per unit aperture area (Goodfriend, 1986).
Conclusions
This study presents the first molecular phylogeny study on the genus Geotrochus and Trochomorpha. The phenotypically identified Sabah Geotrochus and Trochomorpha species do not congruent with the phylogenetic relationships. This incongruency is due to the homoplasy of upper surface sculpture which is used as the diagnostic character of the two genera. The coarser shell character may be an adaptation of the land snails to highland habitat with a more humid condition in the area. Besides, species at the lower elevation habitat tend to has a smaller shell. From the finding above, we concluded that the upper shell sculpture and shell size could not be used for the delimitation of Sabah Geotrochus and Trochomorpha. Hence, the current taxonomy of the two genera need further revision, and the future attempt should consider more samples that cover the entire distribution of the two genera.
Supplemental Information
The maps of distribution for (A) Geotrochus and (B) Trochomorpha species based on the records with coordinate information obtained from Global Biodiversity Information Facility (GBIF)
The data was extracted from GBIF on September 1, 2020, and the distribution map was generated by using the records with coordinates information.
Results of ModelTest for each partition of DNA sequencing alignment (16S, COI, and ITS), and Best partition scheme and substitution models for concatenated sequence data matrix
Each of the five partitions, namely, codons of COI, namely, 1st, 2nd and 3rd codon positions of COI, 16S rDNA, and ITS-1, was tested for molecular evolution via ModelFinder (Kalyaanamoorthy et al., 2017) and partition models (Chernomor, Von Haeseler & Minh, 2016) based on the both AIC and BIC that built into IQ-Tree v.1.6.7 (Nguyen et al., 2015; Trifinopoulos et al., 2016). We limited the candidate models to the six models that are available in MrBayes analysis, namely, JC, F81, K80, HKY, SYM and GTR.
DNA sequences data and input files used for phylogenetic analysis in CIPRES
A concatenated DNA data matrix for 16S, COI, and ITS Sequences for 36 taxa. DNA sequences alignment in FASTA format. Position 1–461: 16S; Position 462–1112: COI; and Position 1113–1918: ITS.
Script for shell morphological data and phylogenetic signal analysis
Script for statistical analyses and Figs. 4 and 5 of the association between shell morphology and environmental variables analysis which followed by the script for phylogenetic signal analyses and Fig. 6 of the phylogenetic signal analyses for shell sculptures and quantitative shell traits.
Shell traits for the ten taxa in the representative phylogenetic tree used for phylogenetic analyses
For the qualitative shell trait –shell surface sculpture types, all the ten taxa with nine species in the phylogenetic tree used for the phylogenetic analysis. However, for the quantitative shell traits, namely, maximum shell height, maximum shell width, maximum aperture height, and maximum aperture width, the tips of the phylogenetic tree represented by the juvenile specimen (i.e. T. thelecoryphe) were excluded.
A maximum-likelihood phylogenetic tree based on the concatenated sequence data matrix
This tree was used for phylogenetic signal analyses together with File S4 and File S5.
Bayesian inference trees of Geotrochus and Trochomorpha species based on each of the 16S, COI and ITS genetic markers
Support values on branches are Bayesian posterior probability (BI) followed by maximum likelihood (ML) bootstrap value. The number shown beside each specimen is same as the specimen number in Table 3, and the specimens with red curly bracket and red arrows are taxa that are incongruent with the clades in the phylogenetic tree estimated based on the concatenated sequences data matrix.
The full dataset of five shell traits of 155 specimens of Trochomorpha and Geotrochus species
The dataset was used for the association between shell morphology and environmental variables analysis with the R script of File S4.