A new Andean treefrog (Amphibia: Hyloscirtus bogotensis group) from Ecuador: an example of community involvement for conservation
- Published
- Accepted
- Received
- Academic Editor
- Tomas Hrbek
- Subject Areas
- Biodiversity, Biogeography, Conservation Biology, Taxonomy, Zoology
- Keywords
- Criptic diversity, Hyloscirtus conscientia sp. nov., Mirra river basin, Montane forest, Northwestern Ecuador
- Copyright
- © 2021 Yánez-Muñoz et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. A new Andean treefrog (Amphibia: Hyloscirtus bogotensis group) from Ecuador: an example of community involvement for conservation. PeerJ 9:e11914 https://doi.org/10.7717/peerj.11914
Abstract
We provide several lines of evidence to delimit a new species of Hyloscirtus and define its phylogenetic position inside the Hyloscirtus bogotensis group. The new species is the sister taxon to Hyloscirtus mashpi and is related to a clade formed by H. alytolylax and a putative new species from the province of El Oro in, southwestern Ecuador. Hyloscirtus conscientia sp. nov. is described from the montane forests of the Mira River basin in the extreme northwestern Ecuador. The new species is characterized as follows: tympanic annulus conspicuous, tip of snout in dorsal view subacuminate, middorsal stripe formed by melanophores larger and less dense, dorsal skin with individual iridophores forming dots, scarcely distributed across dorsum. Our study also highlights the importance of the Mira River Valley as a biogeographic barrier; suggesting research efforts north and south of the valley are likely to reveal additional endemic cryptic diversity. Finally, our partnership with Reserva: The Youth Land Trust, Rainforest Trust and EcoMinga Foundation has produced a novel and meaningful way to connect young people with biodiversity discovery and habitat conservation.
Introduction
Hyloscirtus Peters 1862 is a genus of stream-breeding treefrogs. Endemic to the Neotropics, it reaches its highest species richness in the northern Andes (Faivovich et al., 2005; Coloma et al., 2012; Frost, 2021). With 37 formally described species, several studies have determined that Hyloscirtus contains four species groups: H. bogotensis group, H. armatus group, H. larinopygion group and H. jahni group (Duellman, 1972, 1973; Faivovich et al., 2005; Rojas-Runjaic et al., 2018). The H. bogotensis group currently includes 17 species distributed across the Andes of Venezuela, Colombia, and Ecuador, and the lowlands of Costa Rica, Panama, Colombia, Ecuador and Peru (Faivovich et al., 2005; Guayasamin et al., 2015; Rivera-Correa, García-Burneo & Grant, 2016; Ron et al., 2018). Apparently, the only putative morphological synapomorphy of the bogotensis group is the presence of wide dermal fringes on fingers and toes (Faivovich et al., 2005); the presence of mental gland (Duellman, 1972) is considered a plesiomorphic character in Cophomantinae (Brunetti et al., 2015; Rivera-Correa, García-Burneo & Grant, 2016; Faivovich et al., 2018). However, numerous studies support the monophyly of the group on the basis of molecular data (Faivovich et al., 2005; Coloma et al., 2012; Guayasamin et al., 2015; Rivera-Correa, García-Burneo & Grant, 2016; Ron et al., 2018).
Species diversity within the H. bogotensis group is far from being fully understood. Guayasamin et al. (2015) revealed the existence of several cryptic lineages in the Hyloscirtus alytolylax species complex from the northwestern Andes of Ecuador. During the last five years, we have increased sampling efforts in northern Ecuador, particularly in the Mira River basin, near the border with Colombia. We have focused on this area because the Mira River has been identified as an important biogeographic barrier for small vertebrates and epiphytic plants (Croat, 2015; Baquero & Zuchan, 2017; Yánez-Muñoz et al., 2018; Reyes-Puig et al., 2020; Brito et al., 2020).
In the course of this field work, we discovered a new cryptic species of Hyloscirtus, previously confused with H. alytolylax, that we describe below. We levereaged our taxonomic work to increase public awareness of the conservation issues surrounding this species and its habitat, and to test novel methods for engaging young people in scientific processes. ‘Reserva: The Youth Land Trust’ held a global contest to name this species which yielded hundreds of creative and thoughtful submissions, including the scientific and common names proposed for the new species described herein. This program exemplifies how engaging the public in scientific processes can be a powerful tool for raising awareness of biodiversity conservation.
Materials and Methods
Ethics statement
We conducted this study under research permits MAE-DNB-CM-2016-0045 and N°MAE-DNB-CM-2019-0120, issued by the Ministerio del Ambiente del Ecuador. We followed the guidelines for use of live amphibians and reptiles in field research (Beaupre et al., 2004), compiled by the American Society of Ichthyologists and Herpetologists, the Herpetologists’ League and the Society for the Study of Amphibians and Reptiles.
Taxon sampling
We examined specimens deposited in the herpetological collections of the Instituto Nacional de Biodiversidad, Quito (DHMECN) and the University of Kansas, Lawrence (KU) (Appendix 1). All museum acronyms follow Sabaj (2016). Our taxonomic description employs several lines of evidence, including external morphological characters, linear morphometry variations, genetic divergence, monophyly, and geographic data. Similar approaches have been useful in recognizing and identifying closely related species of small vertebrates in the northern Andes (Yánez-Muñoz et al., 2018; Brito et al., 2020; Reyes-Puig et al., 2020).
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved, and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: LSID urna:lsid:zoobank.org:pub:DCC900F2-B242-4357-9B85-D62C659EE135. LSID urn:lsid:zoobank.org:act:38AD2D96-56EF-4AE0-AD98-6E4057CCA0D7.
Field work
Herpetological searches were conducted using visual encounter surveys, following methods proposed by Crump & Scott (1994). Field work was conducted in the province of Carchi, Ecuador, during joint expeditions of the Instituto Nacional de Biodiversidad (INABIO) and Ecominga Foundation. We visited the following localities: (a) Río Pailón (0.983578397 N; −78.29651972 W; 1,495 m) on 6–11 November 2017, 19–25 August 2018, 15–20 September 2020 and 15–20 November 2020; (b) Río Baboso, Reserva Étnica Forestal Awa (0.923881 N, −78.432130 W; 1,117 m), 16–22 July and 1–5 December 2019; (c) El Guapilal, Cerro Negro Reserva Dracula (0.891944 N, −78.20308 W, 1,640–1,750 m), 13–18 April 2020 and 22–27 September 2020. (d) Bosque Comunal la Esperanza (0.939753 N, −78.242000 W, 1,699–1,980 m), 22–31 March 2021.
Collected individuals were photographed alive and euthanized with benzocaine. A sample of muscle tissue was extracted and preserved in 95% ethanol. Specimens were fixed in 10% formalin and preserved in 75% ethanol.
Morphologic data and analysis
Species description follows the general format proposed by Duellman & Hillis (1990) and Kizirian, Coloma & Paredes-Recalde (2003). Webbing formulae are described following Savage & Heyer (1967), with modifications by Myers & Duellman (1982). Sex and age were determined by identification of secondary sexual characteristics (nuptial pads and vocal slits in adult males) and direct gonad inspection through dorsolateral incisions. Morphometric measurements were taken with an electronic caliper (precision ±0.01 mm, rounded to 0.1 mm), following descriptions and comments by Guayasamin et al. (2015). The following morphological measurements were taken in 50 males (Supplemental Material 1), corresponding to four species in the Hyloscirtus alytolylax species complex: snout-vent length (SVL), head length (HL), head width (HW), upper eyelid width (EW), interorbital distance (IOD), inter-nostril distance (IND), nostril eye distance (NED), eye diameter (ED), tympanum diameter (TD), hand length (HaL), tibia length (TL), and foot length (FL). We reduced the dimensionality of the morphometric data set, using a Principal Component Analysis (PCA), in order to determine the variability of a data set and ordered them by importance of their variables. Initially, data normality was determined using the Shapiro-Wilk test. As a second step, the influence of the SVL on the rest of the variables and its significance were determined, through a simple linear regression; for those variables where the SVL was significant (according to the analysis of covariance), the residuals of the linear regression were extracted, and the PCA was conducted with the residuals (Supplemental Material 1). The main components with eigenvalues greater than unity and that explain the highest percentage of the variation were used (Table 1).
| Morphological variable | Components | |||
|---|---|---|---|---|
| PC 1 | PC 2 | PC 3 | PC 4 | |
| HW | 0.20728 | −0.039829 | 0.11563 | 0.33942 |
| HL | 0.36365 | −0.2772 | 0.79021 | −0.2281 |
| NED | −0.0043312 | −0.093427 | 0.082997 | −0.31115 |
| IND | 0.037503 | −0.055284 | 0.091971 | 0.037526 |
| IOD | 0.13103 | 0.057463 | 0.12493 | 0.80219 |
| EW | 0.0014943 | 0.11548 | 0.26451 | 0.19189 |
| TD | −0.017409 | 0.06092 | −0.016531 | 0.095268 |
| ED | 0.081547 | −0.056741 | 0.025114 | −0.069391 |
| TL | 0.62551 | −0.57726 | −0.42938 | 0.046531 |
| HaL | 0.34742 | 0.49207 | 0.15975 | −0.044192 |
| FL | 0.53599 | 0.55756 | −0.22469 | −0.19029 |
| Charge extraction sums | ||||
| Total | 2.3096841 | 0.183752 | 0.984511 | 0.669702 |
| % of Variance | 39.381 | 16.348 | 12.295 | 8.5572 |
| % accumulated | 39.381 | 55.729 | 68.024 | 76.5812 |
We also evaluated quantitative differences between Hyloscirtus mashpi Guayasamin et al. (2015) and the new species using univariate t-tests for independent samples for normal data, and Wilcoxon–Mann Whitney tests for differences in non-normal data. We tested data distribution with Shapiro–Wilk normality tests (Table 2).
| Character | Shapiro-Wilk normality test p (α 0.05) | |
|---|---|---|
| Hyloscirtus conscientia | Hyloscirtus mashpi | |
| SVL | 0.1108 | 0.7421 |
| HW | 0.3272 | 0.1879 |
| HL | 0.04422* | 0.8416 |
| NED | 0.396 | 0.4064 |
| IND | 0.7067 | 0.04807* |
| IOD | 0.6504 | 0.2666 |
| EW | 0.4276 | 0.3608 |
| TD | 0.6723 | 0.09691 |
| ED | 0.04849* | 0.8038 |
| TL | 0.972 | 0.1008 |
| HaL | 0.9366 | 0.7419 |
| FL | 0.1776 | 0.4987 |
Note:
Statistical analyses were conducted using the statistical package Past® (Hammer, Harper & Ryan, 2001).
Call analysis
Recordings were obtained at Guapilal, Cerro Oscuro (Reserva Dracula) and El Baboso, province of Carchi, Ecuador, between 19:00–22:00 h in October and November 2019 by Mario H. Yánez-Muñoz and Juan P. Reyes-Puig. Calling individuals were recorded while perched on vegetation ca. 2 m above ground and close to streams. Vocalizations were obtained with an Olympus WS-802 digital recorder at a sampling frequency of 44.1 kHz and 16 bits resolution. Calls were analyzed usingRaven®1.5 (Bioacoustics Research Program, 2014), configured as a Hann-type window with 80% overlap and a size of 512 samples (11.6 ms). Figures of oscillograms and spectrograms were generated in R version 3.6.3 (R Development Core Team, 2019), using packages tuneR (Ligges et al., 2018) and seewave (Sueur, Aubin & Simonis, 2008), with Hann window at 90% overlap with a size of 512 samples of the fast Fourier transform (FFT) and a spectral limit of 10 kHz. We used values of the analyzed parameters to calculate measures of central tendency (means), and dispersion (maximum, minimum, and standard deviation). Definitions, terms, and measurements follow, Köhler et al. (2017) and Sueur (2018). Calls of species of the Hyloscirtus bogotensis group were obtained from digital databases of Instituto Nacional de Biodiversidad, Quito (DHMECN) and the University of Kansas, Lawrence (KU), as well as acoustic information published by Guayasamin et al. (2015) (Table 3). Analog files recorded in magnetic tape format were available and digitized following the procedures proposed by Batallas & Yánez-Muñoz (2020).
| Variables | Hyloscirtus conscientia sp.nov | Hyloscirtus mashpi | Hyloscirtus alytolylax | Hyloscirtus sp.1 | Hyloscirtus sp.2 | Hyloscirtus palmeri | Hyloscirtus simmonsi |
|---|---|---|---|---|---|---|---|
| N = 1/8/16 | N = 4/83/162 | N = 1/10/88 | N = 1/3/18 | N = 1/6/61 | N = 1/16/34 | N = 3/16/61 | |
| Voucher | DHMECN 15004 | Not available | Recording tape KU 826 | DHMECN 3831 | DHMECN 14897 | DHMECN 7988 | Recording tape KU 765 |
| Locality | Cerro Negro (Carchi) | Mashpi (Pichincha) | Mindo (Pichincha) | El Oro | Río baboso (Carchi) | Tobar Donoso (Carchi) | Cauca (Colombia) |
| Type | 1 | – | 1 | 1 | 2 | 2 | 1 |
| Dominant frequency (kHz) | 2.93–3.10 (2.97 ± 0.07) | 2.84–2.99 (2.90 ± 0.07) | 2.58–2.93 (2.75 ± 0.07) | 2.63–3.10 (2.90 ± 0.12) | 2.15–2.24 (2.2 ± 0.02) | 2.07–2.24 (2.17 ± 0.06) | 2.58–3.10 (2.82 ± 0.14) |
| Call duration (ms) | 470–632 (551 ± 114.55) | 330.9–380.2 (353 ± 11.55) | 422–715 (538.10 ± 91.18) | 455–522 (497 ± 49.79) | 255–479 (358 ± 109.91) | 134–2405 (656.69 ± 514.19) | 375–542 (484.88 ± 55.79) |
| Notes per call | 5–6 (5.50 ± 1.49) | 2–3 | 7–12 (8.80 ± 1.62) | 6–7 (6.33 ± 0.58) | 2–3 (2.5 ± 0.55) | 1–4 (2.31 ± 0.79) | 3–4 (3.81 ± 0.40) |
| Note duration (ms) | 16–35 (26 ± 6.13) | – | 8–29 (22.74 ± 4.86) | 19–30 (24.83 ± 2.53) | 23–33 (27.47 ± 2.64) | 74–185 (130.47 ± 36.30) | 26–54 (40.56 ± 6.47) |
| Time between notes (ms) | 60–109 (90.89 ± 18.30) | 115–162 (139 ± 13) | 35–60 (43.67 ± 5.42) | 43–87 (65.12 ± 11.11) | 172-208 (191.67 ± 14.74) | 114–702 (292.45 ± 172.80) | 101–134 (117.69 ± 7.64) |
| Notes per second (rate) | 7.69–11.76 (8.81 ± 1.43) | 0.17–0.50 (0.36 ± 0.99) | 13.16–16.95 (15.18 ± 0.89) | 8.93–14.29 (11.32 ± 1.48) | 4.20–5.13 (4.58 ± 0.36) | 1.20–4.20 (2.73 ± 0.90) | 5.92–7.52 (6.34 ± 0.32) |
| Source | This work | Guayasamin et al., 2015 | This work | This work | This work | This work | This work |
Note:
The abbreviations used in the parameters correspond to: kHz, Kilohertz; ms, milliseconds. Of the values calculated using descriptive statistics, the following are detailed: minimum–maximum (mean ± standard deviation). In the analyzed sample, we indicate: number of specimens/calls/notes as N = X/X/X.
Genetic sampling
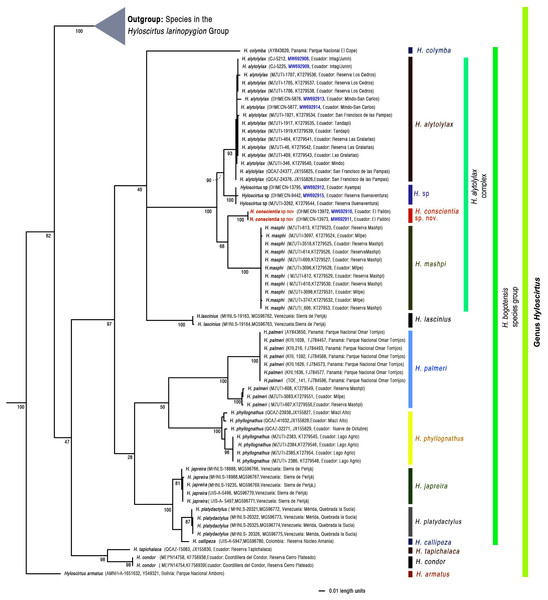
We generated eight new sequences for the mitochondrial 16S gene (see Fig. 1), following the primers and protocols described in Guayasamin et al. (2015). The new sequences were aligned with all those available for Hyloscirtus in GenBank (http://www.ncbi.nlm.nih.gov/genbank), originally published by Faivovich et al. (2005), Crawford, Lips & Bermingham (2010), Coloma et al. (2012), Almendáriz et al. (2014), Guayasamin et al. (2015), and Ron et al. (2018).
Figure 1: Evolutionary relationships of species in the genus Hyloscirtus inferred using Maximum likelihood, based on the mitochondrial gene 16S.
Each terminal is followed by its corresponding museum number, Genbank accession code, and locality. Sequences generated in this study are in blue.Phylogenetic analysis
Sequences were aligned using MAFFT v. 7 (Katoh & Standley, 2013) with the Q-INS-i strategy. Maximum likelihood (ML) trees were estimated using GARLI 2.01 (genetic algorithm for rapid likelihood inference; Zwickl, 2006). GARLI uses a genetic algorithm that finds the tree topology, branch lengths and model parameters that maximize lnL simultaneously (Zwickl, 2006). Individual solutions were selected after 10,000 generations with no significant improvement in likelihood, with the significant topological improvement level set at 0.01. The final solution was selected when the total improvement in likelihood score was lower than 0.05 compared to the last solution obtained. Default values were used for other GARLI settings, as per recommendations of the developer (Zwickl, 2006). Bootstrap support was assessed via 1000 pseudoreplicates under the same settings used in tree search.
We note that our inferred phylogenetic tree is based on a single mitochondrial marker. Although it is well established that mitochondrial genes are informative when resolving recent evolutionary relationships (Avise & Ayala, 2017), it is also true that sometimes gene trees do not agree with the true species tree (Degnan & Rosenberg, 2009). Thus, our inferred tree should be seen as a hypothesis to be tested with additional gene markers.
Results
Phylogenetic relationships
The inferred topology (Fig. 1) agrees with Guayasamin et al. (2015), and differences are mostly explained by a more complete sampling in the Hyloscirtus alytolylax complex. The optimal phylogenetic tree recovered the new species as the sister taxon to H. mashpi and related to a clade formed by H. alytolylax and a putative new species from the southwestern Ecuador province of El Oro (Fig. 1). The uncorrected p genetic distance between the new species and its closest relatives are summarized in Table 4.
| H. conscientia | H. sp.1 | H. alytolylax | H. mashpi | H. colymba | H. lascinius | |
|---|---|---|---|---|---|---|
| H. conscientia | 0.00 | 5.72 | 5.33–5.60 | 6.25–6.38 | 17.01 | 11.74–11.88 |
| H. sp. 1 | 0.00 | 2.73–2.99 | 6.51–6.64 | 15.82 | 11.99–12.13 | |
| H. alytolylax | 0.00–0.39 | 6.39–6.66 | 15.17–15.43 | 10.82–11.21 | ||
| H. mashpi | 0.00–0.26 | 16.74–16.87 | 12.68–12.81 | |||
| H. colymba | 0.00 | 15.73–15.86 | ||||
| H. lascinius | 0.00 |
Note:
Values are presented as percentual distances calculated from uncorrected p values.
Systematic accounts
Hyloscirtus conscientia sp. nov.
LSID urna:lsid:zoobank.org:pub:DCC900F2-B242-4357-9B85-D62C659EE135.
LSID urn:lsid:zoobank.org:act:38AD2D96-56EF-4AE0-AD98-6E4057CCA0D7
Proposed standard Spanish name: Rana nubular torrenticola de Chical
Proposed standard English name: Chical nubulous Stream-Frog
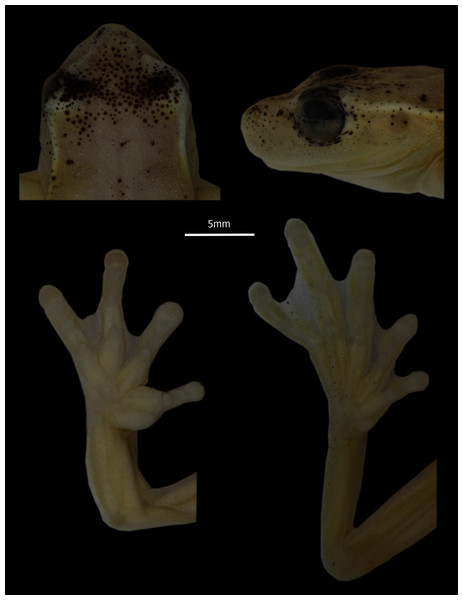
Holotype (Figs. 2, 3). DHMECN 13973, adult male collected at Reserva Dracula (0.983578397; −78.29651972 , 1,495 m), Sector El Pailón Chico, province of Carchi, Republic of Ecuador, on 08 November 2017, by Mario H. Yánez-Muñoz, Juan P. Reyes-Puig and Fausto Recalde.
Figure 2: Dorsal, ventral and profile views of Hyloscirtus conscientia sp. nov., male adult (DHMECN 13973).
Photographs MYM.Figure 3: Detail of head in dorsal and profile view, and hands and feet of Holotype of Hyloscirtus conscientia sp. nov., male adult (DHMECN 13973).
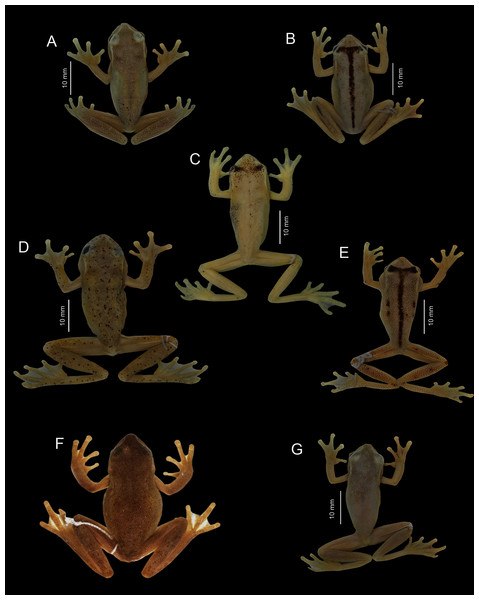
Photographs MYM.Paratypes (Fig. 4). DHMECN 13968–13972, DHMECN 13974–13977, same data as the holotype; DHMECN 15004, adult male, collected at Reserva Dracula (0.891944 N, −78.20308 W, 1,750 m), Sector El Guapilal, Cerro Negro, province of Carchi, Republic of Ecuador, on 23 September 2019, by Mario H. Yánez-Muñoz and Juan P. Reyes-Puig; DHMECN 15138–15140, DHMECN 15142, same data as DHMECN 15004, but collected on 13 April 2019, by Daniela Franco-Mena and Juan P. Reyes-Puig. DHMECN 16114–16114, females, Bosque de la Comuna La Esperanza, province of Carchi, Republic of Ecuador (0.939753 N, −78.242000 W, 1,699m), on 24 March 2021, by Mario H. Yánez-Muñoz, Juan P. Reyes-Puig and Miguel Urgiles-Merchán.
Figure 4: Variation of type series of Hyloscirtus conscientia sp. nov. in preservative.
First row, from left to right: DHMECN 15140, DHMECN 15139, DHMECN 13973, Holotype, DHMECN 13974. Second row, from left to right: DHMECN 13971, DHMECN 15004, DHMECN 15138, DHMECN 13969. Photographs by MYM.Generic placement. We assign the new species to the genus Hyloscirtus Peters, 1882, as defined by Faivovich et al. (2005) and Rojas-Runjaic et al. (2018), and to the H. bogotensis species group (sensu Duellman, 1972; Faivovich et al., 2005; Rojas-Runjaic et al., 2018) based on its phylogenetic position (Fig. 1) and morphology (i.e., wide dermal fringes on fingers and toes).
Diagnosis. Hyloscirtus conscientia sp nov. is characterized by the following combination of characters: (1) adult males small (SVL 29.6–33.3 mm, mean = 31.0 ± 1.0, n = 14), females (SVL 34.7–40 mm, mean = 37.3 ± 2.6mm, n = 3); (2) body relatively slender; (3) snout subacuminate in dorsal view and rounded in lateral view; (4) in life, dorsum usually pale yellowish-green, usually with a thin brown mid-dorsal stripe; (5) axillar and inguinal regions light yellowish-green; (6) mental gland present in males, pigmented in some individuals; (7) upper lip lacking white stripe; (8) parietal peritoneum white, visceral peritonea transparent; (9) iris grayish-brown to copper brown with thin black reticulation; (11) nuptial pad absent; (13) tympanic membrane pigmented as surrounding skin; tympanic annulus rounded and visible; (14) cream supraocular, supratympanic, and canthal stripes usually present; brown interorbital stripe usually present; (15) ulnar fold and tarsal stripe absent or inconspicuous; (16) calcar tubercle absent; (17) supracloacal fold low, with few iridophores; (18) low tubercles scattered around and below cloaca; (19) bones in life white; (20) elliptical prepollex not modified as a projecting spine; (21) dentigerous processes of vomers prominent, slightly curved, with a discernible gap and 10–14 teeth each; and (22) advertisement call with 5 or 6 notes, call duration = 470–632 ms, and dominant frequency = 2.93–3.10 kHz.
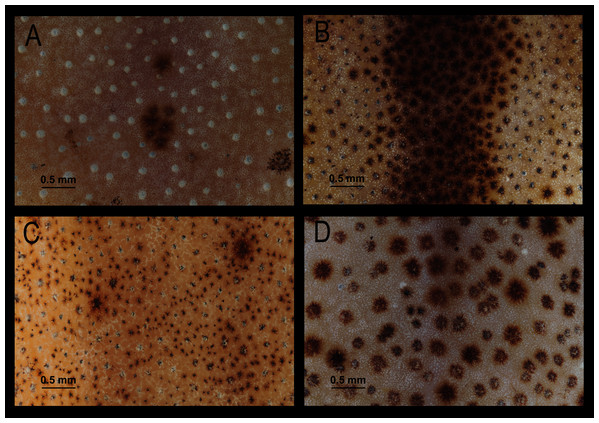
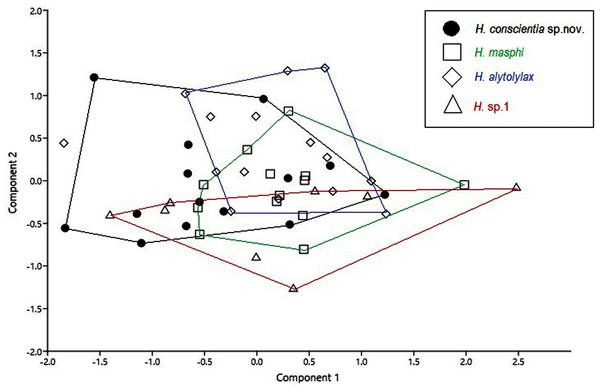
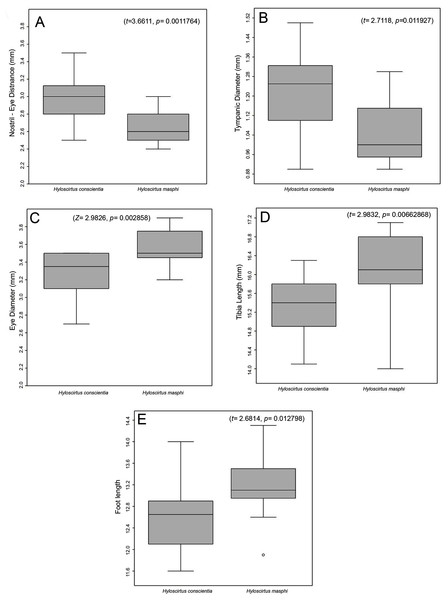
Comparison with similar species (Figs. 5, 6, 7, 8, 9, 10). Hyloscirtus conscientia sp. nov. is most similar to its sister species, H. mashpi Guayasamin et al. (2015), H. alytolytax (Duellman, 1972) and a new candidate species from southwestern Ecuador (Figs. 5, 6, 7). The variability in the morphological space (Fig. 8) was only explained in 39.89% by Principal Component 1, the highest loads were the measurements of the hind limb (FL + TL) (Table 1). There is a considerable degree of overlap among four species included in the analysis. There are, however, some statistical differences; H. mashpi has a significantly larger eye diameter than H. conscientia sp. nov., as well as longer TL and HaL; and H. conscientia sp. nov. has significantly longer TD and NED than H. mashpi (Table 5, Fig. 9). Externally, Hyloscirtus mashpi differs from H. conscientia sp. nov. as follows (characters of H. conscientia sp. nov. in parentheses): ON/DIO ratio lower (80% in H. mashpi vs. 91% in H. conscientia sp. nov.), tympanic annulus inconspicuous (conspicuous), tip of snout in dorsal view truncate (rounded, Figs. 6B, 6C), mid-dorsal stripe formed by melanophores smaller and more densely packed (mid-dorsal stripe formed by melanophores larger and less dense), dorsal skin with individual iridophores packed on top of melanophores but never forming dots (iridophores forming dots, scarcely distributed across dorsum; Figs.7B, 7C).
Figure 5: Comparisons in preservative of some species of the Hyloscirtus bogotensis group.
(A) H. alytolylax, DHMECN 5875; (B) H. mashpi, DHMECN 4289; (C) H. conscientia sp. nov., Holotype DHMECN 13973; (D). H. sp. candidate confirmed, DHMECN 1976; (E) H. sp. candidate not confirmed, DHMECN 0173; (F) H. simonsi, Holotype KU 169554; (G) H. candidate not confirmed, DHMECN 4750. Red arrows indicate the contour of the tip of the snout. Photographs MYM.Figure 6: Comparison of tip snout in dorsal view of some preserved specimens of species in the Hyloscirtus bogotensis group.
(A) H. alytolylax, DHMECN 5875; (B) H. mashpi, DHMECN 4289; (C) H. conscientia sp. nov., Holotype DHMECN 13973; (D). H. sp. candidate confirmed, DHMECN 1976; (E) H. sp. candidate not confirmed, DHMECN 0173; (F) H. simonsi, Holotype KU 169554; (G) H. candidate not confirmed, DHMECN 4750. Red arrows indicate the contour of the tip of the snout. Photographs MYM.Figure 7: Comparison of dorsal skin texture of some preserved specimens of species in the Hyloscirtus bogotensis group.
(A) H. alytolylax, DHMECN 5875; (B) H. mashpi, DHMECN 7115; (C) H. sp. candidate confirmed, DHMECN 1976; (D) H. conscientia sp. nov., DHMECN 15142. Photographs by Lou Jost.Figure 8: Principal component analysis from morphometric variables of the species of the Hylocirtus alytolylax complex.
Figure 9: (A–E) Comparison of morphometric data between Hyloscirtus conscientia sp. nov. and H. mashpi.
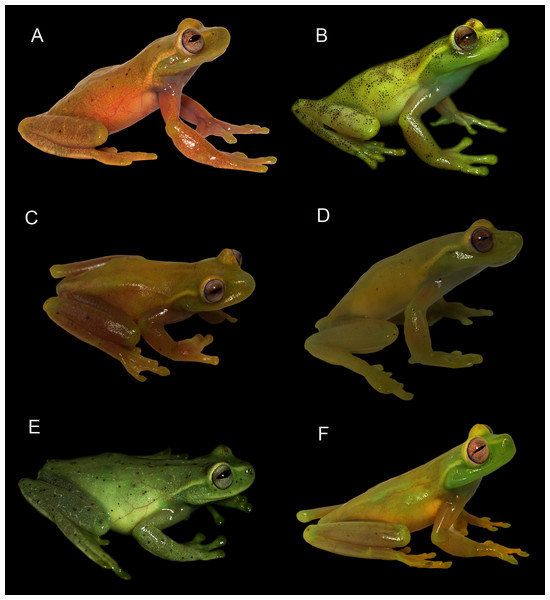
Figure 10: Comparison of live coloration of some species of the Hyloscirtus bogotensis Group.
(A) H. alytolylax, male, not collected, Estación Experimental La Favorita, province of Pichincha; (B) H. mashpi, male, DHMECN 4289, Reserva Mashpi, province of Pichincha; (C) H. conscientia, male, holotype DHMECN13973, Río Pailón Chico, Reserva Dracula, province of Carchi; (D) H. conscientia sp. nov., male, paratype DHMECN 13972, Río Pailón Chico, Reserva Dracula, province of Carchi; (E) H. sp. candidate confirmed, (DHMECN 3743); Reserva Buenaventura, province of El Oro; (F) H. sp. candidate not confirmed, El Baboso, province of Carchi. Specimens without scale. Photographs: MYM (A–E) and Mateo Vega-Yanez (F).| Character | Hyloscirtus conscientia | Hyloscirtus mashpi | t | p | Z | p |
|---|---|---|---|---|---|---|
| SVL | 29.6–33.3 (14) 31.0 ± 1.0 | 28.7–32.2 (13) 31.3 ± 1.2 | 0.86808 | 0.39361 | – | – |
| HW | 9.8–10.8 (14) 10.2 ± 0.3 | 9.5–11 (13) 10.3 ± 0.5 | 0.90816 | 0.37246 | – | – |
| HL | 10.0–12.2 (14) 10.7 ± 0.5 | 9.9–11.7 (13) 10.7 ± 0.5 | – | – | 0.073001 | 0.94181 |
| NED | 2.5–3.5 (14) 3.0 ± 0.3 | 2.4–3.0 (13) 2.6 ± 0.2 | 3.6611 | 0.0011764* | ||
| IND | 2.0-3.0 (14) 2.5 ± 0.3 | 2.1-2.8 (13) 2.5 ± 0.2 | – | – | 0.024748 | 0.98026 |
| IOD | 2.8–3.7 (14) 3.3 ± 0.2 | 2.7–3.7 (13) 3.3 ± 0.3 | 0.27696 | 0.78409 | – | – |
| EW | 2.2–3.1 (14) 2.6 ± 0.3 | 2.0–2.9 (13) 2.4 ± 0.3 | 1.5579 | 0.13182 | – | – |
| TD | 0.9–1.5 (14) 1.2 ± 0.2 | 0.9–1.3 (13) 1.1 ± 0.1 | 2.7118 | 0.011927* | – | – |
| ED | 2.7–3.5 (14) 3.3 ± 0.2 | 3.2–3.9 (13) 3.6 ± 0.2 | – | – | 2.9826 | 0.0028578* |
| TL | 14.1–16.3 (14) 15.3 ± 0.6 | 14.0–17.1 (13) 15.1 ± 0.8 | 2.9832 | 0.0062868* | – | – |
| HaL | 8.4–10.4 (14) 9.3 ± 0.5 | 8.6–10.4 (13) 9.6 ± 0.5 | 1.3187 | 0.19924 | – | – |
| FL | 11.6–14.0 (14) 12.6 ± 0.6 | 11.9–14.3 (13) 13.2 ± 0.6 | 2.6814 | 0.012798* | – | – |
Notes:
For each character, values t and z, and p are given, after rank, sample size (in brackets) and mean ± standard deviation for each species. Measurements: Snout-Vent length (SVL), Tibia length (TL), Foot length (FL), Head length (HL), Head width (HW), Eyelid width (EW), Interorbital distance (IOD), Inter-narinal distance (IND), Eye-Narina distance (EN), Eye diameter (ED), Tympanic diameter (TD), Hand length (HaL).
Hyloscirtus alytolylax differs from H. conscientia sp. nov. as follows (characters of H. conscientia sp. nov. in parenthesis): legs usually with thin white dorsal reticulum (absent; Fig. 10), ulnar and tarsal folds clearly present and covered by iridophores (absent or weak with faint white coloration), mid-dorsal stripe usually absent (thin mid-dorsal stripe usually present), iridophores forming abundant dots (iridophores forming scarcely distributed dots) (Figs. 7A, 7D). Two other species of the H. bogotensis group occur in the Pacific lowlands and western Andean slopes of Colombia and Ecuador: Hyloscirtus palmeri (Boulenger, 1908) and H. simmonsi (Duellman, 1989). Hyloscirtus palmeri clearly differs from H. conscientia sp. nov. by having calcars, while H. simmonsi differs by lacking cream supraocular, supratympanic, and canthal stripes (Fig. 6F). For a summary of traits that differentiate the new species from other closely related taxa, see Appendix 2.
Description of holotype (Figs. 2, 3). Adult male 31.7 mm SVL. Body relatively slender (Fig. 2). Head longer than wide (HL 33% SVL; HW 33% SVL). Snout subacuminate in dorsal view and rounded in lateral views; canthus rostralis distinct, slightly concave; loreal region slightly concave; lips rounded, not flared (Fig. 3). White canthal stripe present, incomplete (Fig. 3). Nostrils not protuberant, directed anterolaterally at level of anterior margin of lower jaw. Internarial region and top of head round. Interorbital distance longer than upper eyelid (IOD 129.63% EW). Eye prominent (ED 8% SVL). Tympanic membrane visible, tympanic annulus evident, rounded, upper portion covered by supratympanic fold (TD 4% SVL). Supratympanic fold starting at posterior end of upper eyelid and reaching posterior margin of insertion of arm (Fig. 2). Supratympanic stripe white. Mental gland present, diamond-shaped, partially covering the gular area and extending about half length of throat (Fig. 2). Dentigerous processes of vomers conspicuous, straight, narrowly separated from each other; each process bears 7 (right) and 8 (left) teeth. Choanae large, elliptical, not concealed by palatal shelf of maxillary arch. Tongue with a rounded outline, attached overall (narrowly free around lateral and posterior margin); vocal slits present, longitudinal, originating on sides of tongue and extending to posterolateral corner of mouth. Vocal sac moderately distensible, evident externally, single, median and subgular. Forearm moderately robust; axillary membrane absent. Outer ulnar fold present. Fingers relatively short, thick, bearing small, ovoid discs; each disc only slightly expandable laterally, and with clearly defined circumferential groove; disc on finger III about the same width as TD. Relative length of fingers I < II < IV < III. Fingers with fleshy dermal fringes; webbing present only between outer fingers; webbing formula II 2–3 III 21/2–2–IV. Subarticular distal tubercle large and elliptical. Supernumerary tubercles present, fleshy and small. Palmar tubercle poorly differentiated. Inner metacarpal tubercle large, thick, elliptical. Broad elliptical prepollex, not modified as a spine. Nuptial excrescences absent. Hind limbs moderately robust; tibia length 49% SVL; foot length 40% SVL. Outer tarsal fold and calcar tubercle absent, with weak tarsal stripe; inner tarsal fold indistinct. Toes relatively short, with thin lateral fringes, bearing discs slightly smaller than those on fingers. Relative length of toes I < II < V < III < IV; extensive toe webbing, formula I 1–11/2 II 11/3–11/2 III 1+– 12/3 IV 2–1V. Inner metatarsal tubercle elongate, elliptical, flat; subarticular tubercles small, round; outer metatarsal tubercle absent; supernumerary tubercles not distinctive. Cloacal opening directed posteroventrally at mid-level of thighs; supracloacal fold present, thick; conspicuous tubercles scattered around and below cloaca. Dorsal skin, gular and pectoral regions flanks smooth; venter aerolate. White parietal peritoneum present.
Coloration of holotype in preservative (Fig. 2). Dorsally yellowish-white, homogeneous. Melanophores scattered on eyelids and nasal region. White line on canthus, external border of upper eyelid and supratympanic fold. Outer edge of heel weakly pigmented white. Ventrally yellowish-white. Iris light brown with thin black reticulation.
Coloration of holotype in life (Figs. 10C). Dorsally homogeneously intense light green. Dorsum and dorsal surfaces of legs with small, well-defined white spots. Canthal surface finely dotted with melanophores. Creamy green canthal line (incomplete) and supratympanic fold. Venter white; throat, gular sac, and pectoral region transparent dark green; ventral surfaces of shanks pinkish green. Iris, creamy pinkish with fine dark brown spots around the pupil.
Measurements of the holotype (in mm). SVL = 31.7; HW = 10.4; HL = 10.4; NED = 2.6; IND = 2.7; IOD = 3.5; EW = 2.7; TD = 1.3; ED = 3.5; TL = 15.7; HL = 9.6; FL = 12.8.
Variation (Figs. 4, 11). Morphometric variation of the type series is presented in Table 6. In preservative (Fig. 4), the new species shows dorsal coloration varying from predominantly yellowish white with scattered little brown spots (DHMECN 15140) to a extremely pigmented dark brown pattern in a yellowish matrix (DHMECN 13969), passing through different combinations of dark brown with pale diffuse areas on limbs and flanks (DHMECN 15138) to partially pigmented with mid-dorsal and interorbital lines formed by brown melanophores (DHMECN 15004), either partially defined (DHMECN 13971) or dispersed in the head on a white matrix (DHMECN 13974). Ventral coloration is immaculate white.
Figure 11: Variation in live coloration of Hyloscirtus conscientia sp. nov.
Individuals from Río Pailón Chico at Reserva Dracula. Not collected specimens, without scale. Photographs by MYM.| Code collection | SEX | SVL | HW | HL | NED | IND | IOD | EW | TD | ED | TL | HaL | FL |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DHMECN13973* | ♂ | 31.7 | 10.4 | 10.4 | 2.6 | 2.7 | 3.5 | 2.7 | 1.3 | 3.5 | 15.7 | 9.6 | 12.8 |
| DHMECN15004 | ♂ | 32.25 | 10.5 | 12.2 | 3.5 | 2.5 | 2.9 | 3 | 0.9 | 3.5 | 16.3 | 10.4 | 14 |
| DHMECN15138 | ♂ | 31.7 | 9.9 | 10.4 | 3 | 2.5 | 3.1 | 2.7 | 1.3 | 3.4 | 15.8 | 9.6 | 12.1 |
| DHMECN15139 | ♂ | 30.5 | 10.8 | 10.6 | 3.1 | 2.7 | 3.5 | 2.5 | 1.2 | 3.2 | 15.8 | 9 | 12.5 |
| DHMECN15140 | ♂ | 33.3 | 10 | 11.1 | 3.5 | 2.4 | 3.7 | 2.6 | 1 | 3.2 | 16.1 | 9.3 | 12.9 |
| DHMECN13968 | ♂ | 29.6 | 10.2 | 11.1 | 3 | 2.9 | 3.3 | 2.2 | 1.1 | 3.5 | 15.4 | 9.2 | 12.8 |
| DHMECN13969 | ♂ | 30.2 | 9.8 | 10.5 | 2.8 | 2.4 | 3 | 2.8 | 1.3 | 3.5 | 14.6 | 10 | 12.8 |
| DHMECN13970 | ♂ | 30.6 | 9.9 | 10.8 | 3 | 2.3 | 2.8 | 2.2 | 1.5 | 3.1 | 15.2 | 8.8 | 12.1 |
| DHMECN13971 | ♂ | 30.2 | 10 | 10.2 | 2.5 | 2.4 | 3.2 | 2.4 | 1.3 | 3.1 | 14.5 | 9.4 | 12.1 |
| DHMECN13972 | ♂ | 30.6 | 10.3 | 10.5 | 2.8 | 2 | 3.3 | 2.2 | 1.1 | 2.7 | 15.2 | 9 | 12.2 |
| DHMECN13974 | ♂ | 31.4 | 10.2 | 10 | 2.9 | 2.6 | 3.1 | 2.4 | 1.5 | 3 | 14.1 | 9.6 | 13 |
| DHMECN13975 | ♂ | 30.7 | 10.7 | 10.6 | 2.8 | 3 | 3.7 | 2.9 | 1.2 | 3.3 | 14.9 | 8.4 | 11.6 |
| DHMECN13976 | ♂ | 30.5 | 10.2 | 11 | 3 | 2.4 | 3.6 | 3.1 | 1.4 | 3.4 | 15.1 | 8.8 | 12.2 |
| DHMECN13977 | ♂ | 30.4 | 9.8 | 10.8 | 3.2 | 2.6 | 3.3 | 2.8 | 1.1 | 3.4 | 15.4 | 9.2 | 12.9 |
| DHMECN16007 | ♀ | 40 | 13.75 | 14.42 | 3.62 | 3.12 | 5.2 | 3.32 | 1.21 | 4.71 | 22.4 | 11.76 | 17.67 |
| DHMECN16114 | ♀ | 37.35 | 12.15 | 12.63 | 2.86 | 2.78 | 4.5 | 2.99 | 1.41 | 3.53 | 19.43 | 12.17 | 17.08 |
| DHMECN16115 | ♀ | 34.68 | 11.35 | 12.47 | 2.84 | 2.66 | 3.81 | 3.06 | 1.63 | 4.2 | 17.22 | 11.13 | 14.62 |
| Rank males | 29.6–33.3 (14) 31.0 ± 1.0 | 9.8–10.8 (14) 10.2 ± 0.3 | 10.0–12.2 (14) 10.7 ± 0.5 | 2.5–3.5 (14) 3.0 ± 0.3 | 2.0–3.0 (14) 2.5 ± 0.3 | 2.8–3.7 (14) 3.3 ± 0.2 | 2.2–3.1 (14) 2.6 ± 0.3 | 0.9–1.5 (14) 1.2 ± 0.2 | 2.7–3.5 (14) 3.3 ± 0.2 | 14.1–16.3 (14) 15.3 ± 0.6 | 8.4–10.4 (14) 9.3 ± 0.5 | 11.6–14.0 (14) 12.6 ± 0.6 | |
| Rank females | 34.7–40 (3) 37.3 ± 2.6 | 11.4–13.8 (3) 12.4 ± 1.2 | 12.5–14.4 (3) 13.2 ± 1.1 | 2.6–3.8 (3) 3.1 ± 0.4 | 2.8–3.6 (3) 2.9 ± 0.3 | 3.8–5.2 (3) 4.5 ± 0.7 | 2.9–3.3 (3) 3.1 ± 0.2 | 1.2–1.6 (3) 1.4 ± 0.2 | 3.5–4.7 (3) 4.5 ± 0.6 | 17.2–22.4 (3) 19.68 ± 2.6 | 11.1–12.2 (3) 11.7 ± 0.5 | 14.6–17.7 (3) 16.5 ± 1.6 | |
Note:
In life (Fig. 11), Hyloscirtus conscientia presents a wide range of dorsal color variation, with several patterns and intensity of brown pigmented melanophores in dorsal surfaces of the head and body, set in a yellowish green matrix, with a white or pale light green supratympanic fold. Some individuals also exhibit a mid-dorsal and interorbital lines composed of dense brown melanophores (Fig. 11). A light lemon-green morph has small brown points scattered on the dorsum, coloration becoming paler on flanks and limbs with scattered little brown spots, similar to other paler and darker intermediate morphs described above (Fig. 11). Iris coloration varies from silver to bronze, light brown, or whitish gray with black thin reticulations along the margin of the eye. Ventral coloration is predominantly white, males present a white to light green vocal sac, ventral surfaces of limbs are whitish-yellow. Field observations suggest that color can become darker or lighter as a form of chromatic crypsis used for camouflage.
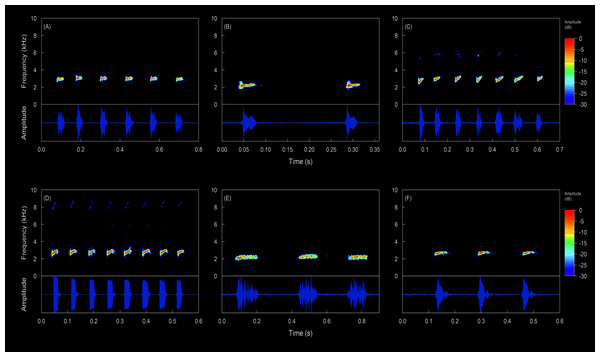
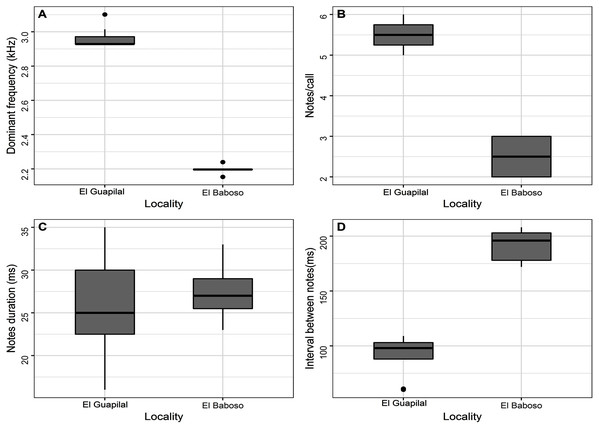
Call description (Figs. 12, 13). The call of Hyloscirtus conscientia consists of a series of notes (five or six notes per call) . Call duration ranges from 470–655 ms. Note duration ranges from 16–35 ms, with intervals between notes from 60–109 ms, emitted at a rate of 7.69–11.76 notes/second (Table 3). Each note has 2–4 pulses. The dominant frequency ranges from 2.93–3.10 kHz. There are no visible harmonics.
Figure 12: Oscillograms and spectrograms of the song of Hyloscirtus conscientia sp. nov. compared with the calls of several species of the Hyloscirtus bogotensis group.
(A) Hyloscirtus conscientia sp. nov. from the Guapilal Reserva Dracula (B) Hyloscirtus sp. 1 from El Baboso; (C) Hyloscirtus sp. 2 from El Oro; (D) H. alytolylax sensu stricto from Mindo; (E) H. palmeri from Tobar Donoso; (F) H. simmonsi from El Cauca-Colombia. For voucher datta see Table 6.Figure 13: Box plot comparing variation of the spectral and temporal parameters of the notes of the Hyloscirtus conscientia sp. nov. of Guapilal, Reserva Dracula and Hyloscirtus sp. from El Baboso.
Hyloscirtus conscientia sp. nov. presents a dominant frequency with values similar to other species belonging to the H. bogotensis group. However, the dominant frequency of H. conscientia sp. nov. is slightly higher than that reported for H. mashpi (2.93–3.10 kHz in the new species; 2.84–2.92 kHz in H. mashpi; Guayasamin et al., 2015; Table 3, Fig. 12). Other differences are the call duration (470–655 ms in H. conscientia sp. nov. and 331–380 ms in H. mashpi; Guayasamin et al., 2015) and the number of notes per call (5 or 6 in H. conscientia sp. nov. 2 or 3 in H. mashpi; Guayasamin et al., 2015). The calls of Hyloscirtus conscientia sp. nov. and H. alytolylax sensu stricto are very similar (Table 3, Fig. 12) and the only non-overlapping trait is the time between notes (60–109 ms in the new species; 35–60 ms in H. alotylylax; see Table 3).
The analyzed calls of the species in the Hyloscirtus bogotensis group exhibit two pattern types. Type 1, where emissions are short, but have multiple notes (explosive emissions) and a high emission rate (Table 6); this type of emission is present in the new species, H. alytolylax, H. mashpi and H. simmonsi (Figs. 12C, 12D, 12F). Type 2 emissions exhibit longer intervals between notes and a low emission rate (Table 6); this type of emission is characteristic of H. palmeri (Fig. 12E).
There is call variation within Hyloscirtus conscientia and nearby populations. For example, the call recorded at the San Juan River subbasin differs from the populations recorded in the Mira River subbasin (Figs. 12A, 12B). Through an analysis of its spectral and temporal parameters, we preliminarily suggest that this variation is an indicator that populations of San Juan River may be a candidate for a new species (Fig. 13; Table 6).
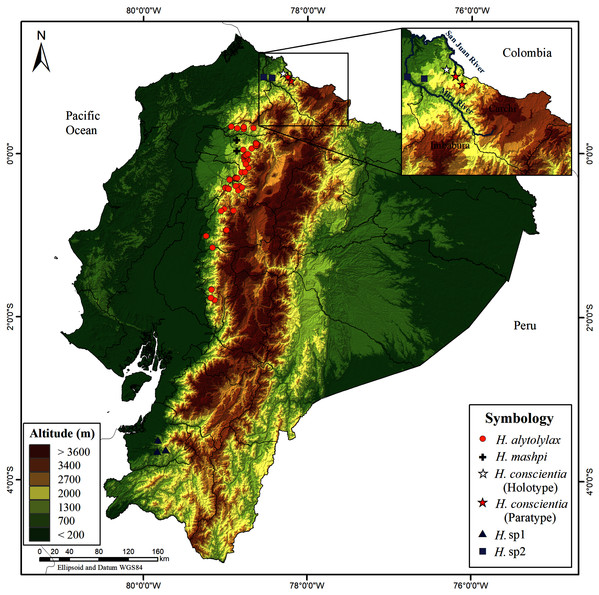
Distribution (Figs. 14). Hyloscirtus conscientia is known only from two nearby locality-points—the Dracula Reserve conservation area, managed by Ecominga Foundation, and Dracula Youth Reserve, managed by Reserva Youth Land Trust. Both reserves are located on the western slopes of the Andes, Carchi Province, Northern Ecuador, at an altitude between 1,495 to 1,750 m. The species is restricted to humid montane forest of the San Juan River drainage, in the Mira basin. Distribution points of Hyloscirtus conscientia are a few kilometers away from the Ecuador-Colombia border; thus, it is likely that H. conscientia is also present in Colombia.
Figure 14: Map of Ecuador showing the distribution of Hyloscirtus conscientia sp. nov. and some species of the H. alytolylax complex.
Natural history. Hyloscirtus conscientia was found along clean-water streams with many waterfalls in montane foothill cloud forest. The riparian forest included trees reaching 20 m high, covered by moss and epiphytes. Frogs were found at night, sitting on or under leaves and branches of Heliconiaceae, shrubs, trees, and ferns, at 30–350 cm above ground. Males were found calling while sitting under leaves in April, September and November. Hyloscirtus conscientia was found sympatric with Hyalinobatrachium sp. aff. valerioi, Espadarana prosoblepon, Rhaebo colomai, Atelopus coynei, and Pristimantis laticlavius.
Tadpoles, metamorphs, and juveniles were found in various months of the year (August, April, and November), suggesting several seasons of reproduction throughout the year. This may be a consequence of the constant high humidity and pluviosity of the Chocó bioregion.
Extinction risk. The new species distribution is limited to two localities in northwestern Ecuador, and both form part of the Dracula Reserve of Ecominga Foundation. The type localities are threatened by mining, cattle ranching, agriculture and deforestation. Field observations suggest that rivers and streams in the area are still free of invasive species, such as the rainbow trout, that have been shown to negatively impact frog communities (Martín-Torrijos et al., 2016; Krynak et al., 2020). Information on the species’ biology, distribution, and ability to cope with threats, such as habitat fragmentation, emerging diseases, global change, and exotic species, is unknown. We suggest that Hyloscirtus conscientia be considered as Data Deficient, following the criteria by the IUCN, pending additional studies. However, if additional information confirm that the species is at risk, it may deserve to be classified under an extinction risk category in the near future.
Etymology. The specific epithet of the new species is a noun in apposition derived from the imperative Latin “conscĭentĭa”. This name was proposed by Carolina Bustillos, a 19 years old Ecuadorian that participated in a global, public contest to select the species name. When explaining the species name, Carolina wrote: “We are at such a critical point in history, where the Earth and its species cannot continue to endure more exploitation and neglect by people. We are all part of this world and we must all have the consciousness to take care of it, to use less water, to use renewable energy sources, to consume less meat …. I think that this little frog should carry that message … the message of being aware of how wonderful this land is, with all its flora and fauna, and of being aware that we should take care of it and be grateful to it.”
The Spanish and English common names of H. conscientia refer to the habitat and location in which the species is found, in the cloud forests near the small town of El Chical, Ecuador. In the Spanish name, the word “Nubular” was explained by Domenique Benítez (14 years old, Ecuador) as a short, simple, and easy-to-memorize word of her own invention. She wrote, “although this word does not exist, it quickly describes the frog’s habitat. I think the next generations should take into account that it is important to conserve species and name new ones in the future.” The word “nubular” makes reference to the constant presence of clouds (Spanish: nubes) on these forests.
Discussion
Our results show another example of cryptic diversity in the Ecuadorian Andes. In recent years, thanks to the incorporation of molecular and acoustic data in taxonomic studies, has facilitated the discovery and description of hidden diversity (Hutter & Guayasamin, 2015; Guayasamin et al., 2015; Brito, Batallas & Yánez-Muñoz, 2017; Páez & Ron, 2019; Guayasamin et al., 2020). These discoveries not only shed light onto the already dazzling diversity of the Andes, but also provide information regarding the importance of some biogeographic barriers that facilitate the isolation and diversification of lineages. One such barrier, supported in this study, is the Mira River Valley (Arteaga et al., 2016; Yánez-Muñoz et al., 2018, 2020; Brito et al., 2020; Guayasamin et al., 2020; Reyes-Puig et al., 2019), which separates the new species from other closely related populations of the Hyloscirtus alytolylax complex.
At regional scale, biodiversity patterns northr of the Mira river show direct influence from mountain ridges on Cerro Golondrinas, an old geologic formation between the San Juan and Mira rivers, main tributaries of the drainage (Yánez-Muñoz et al., 2020). This mountain barrier and the deep canyon formed by the Mira river promoted conditions for isolation and speciation, of species with low vagility in northwestern Ecuador, mainly in areas between the Mira and Esmeraldas rivers (Brito et al., 2020; Yánez-Muñoz et al., 2020).
Our inferred phylogeny is congruent with other studies that support the monophyly of the Hyloscirtus bogotensis species group (Rojas-Runjaic et al., 2018, Guayasamin et al., 2015). This work shows high support for a clade formed by species of the Hyloscirtus alytolylax complex, including H. mashpi, H. conscientia, and a new candidate species from southwestern Ecuador. Bioacoustic evidence showed differences between populations of Hyloscirtus located in different tributary systems of the Mira basin (Mira and San Juan Rivers subbasins), leading us to refrain from including this material as part of H. concientia. Further analyses are necessary to understand the evolutionary relationships of the populations found at El Baboso, in the province of Carchi province, and Alto Tambo in the province of Esmeraldas. Based on coloration pattern analyses, populations from El Baboso (Mira River subbasin) are more similar to H. mashpi than to H. conscientia, showing a distinctive mid-dorsal band composed by melanophores (usually absent in H. conscientia). Females from the Alto Tambo populations, in the Santiago River basin of Esmeraldas province, present a truncate snout tip and males have distinct calls (Figs. 6 and 13). We consider this population as an unconfirmed candidate species.
Community involvement and conservation
Dracula Youth Reserve, one of the type localities of Hyloscirtus conscientia, is an ongoing project by Reserva: The Youth Land Trust to create an entirely youth-funded reserve, in partnership with Rainforest Trust and EcoMinga Foundation. Since September 2019, people aged 26 and younger have been contributing to the conservation of this species’ habitat through individual donations, youth-led online fundraisers, and letters in support of conservation. In honor of these contributions, we invited youth to name this species through a free, online contest hosted on the Reserva: The Youth Land Trust website.
The contest was open to entrants aged 26 and younger throughout the month of September 2020. Participants were given a photo gallery and educational resources about the species and were allowed to submit a specific epithet, an English or Spanish name, and a justification for their choice in English or Spanish. We received 622 unique submissions from 36 countries, with an average participant age of 14.5. In the preliminary round of judging, each entry was read by four of 15 volunteer reviewers, and each entry that received at least two votes proceeded to the next round of judging. The final 54 entries were judged via video call by a panel of judges whose areas of expertise range from international conservation to space exploration: Ellen Stofan, Robert Ridgely, Georgie White, Luis E. Baquero, Grethel Aguilar, and Paula Kahumbu. Judges unanimously selected the winning specific epithet and assembled Spanish and English names from common themes found in 40 of the contest’s entries.
The inclusion of a judging panel and expectation that entrants would review educational materials appears to have impacted the seriousness of responses, which were overwhelmingly positive, educated, and thoughtful attempts to offer a name for the species. The geographic range of participants (627 entries of 36 counties in South America, North America, Africa, and Europe) indicates that engaging the public in the naming of new species can be an effective method to educate those outside the scientific community, especially youth, on the importance of environmental issues and motivate communities both locally and globally to support conservation.
Conclusions
We provide several lines of evidence to delimit a new species of Hyloscirtus and define its phylogenetic position inside the Hyloscirtus bogotensis group. Our study also highlights the importance of the Mira River Valley as a conspicuous biogeographic barrier; therefore, research efforts north and south of the valley are likely to reveal additional endemic cryptic diversity. Finally, our partnership with Reserva: The Youth Land Trust, Rainforest Trust and EcoMinga Foundation has produced a novel and meaningful way to connect young people with biodiversity discovery and habitat conservation.
| Hyloscirtus alytolylax (15): Ecuador: Imbabura (2): Concesión Llumiragua, DHMECN 13281; Río Naranjal, DHMECN 3518–19; Pichincha (13): Chiriboga, Estación Experimental "La Favorita" DHMECN 999; Las Tolas DHMECN 4338–39, DHMECN 5815; Lloa Mindo- San Carlos, DHMECN 5875–77; Reserva las Gralarias DHMECN 10314–15; Saragoza la Union -Rio Cinto DHMECN 5878–79, DHMECN 7377–7378; Reserva Maquipucuna, QCAZ67790–67803. Hyloscirtus mashpi (9): Ecuador: Pichincha (9): Bosque Protector Mashpi, DHMECN 4288–94, DHMECN 7115–16. Hyloscirtus palmeri (12): Ecuador: Carchi (4): Destacamento Militar, Tobar Donoso, DHMECN 7988; Estero 1 Sendero Awa, Tobar Donoso, DHMECN 6684; Río San Juan, Tobar Donoso, DHMECN 12672, 6685; Esmeraldas (8): Río Bogotá DHMECN 3214; Reserva Biológica Canandé DHMECN 2941–44, DHMECN 9548–49; Pichincha (1): Saguangal, DHMECN 4462; Bosque Protector Mashpi, DHMECN 4295. Hyloscirtus simonsi (2): Colombia: Dpto. del Valle, Río Calima, 1.5 km (by road) W, KU 169554 (Holotype), KU 169555. Hyloscirtus sp.1 (9): Ecuador: El Oro (2) Cascadas de Manuel DHMECN 11510; El Birón, DHMECN 13795; Limón Playa, DHMECN 10782; Reserva Biológica Buenaventura, DHMECN 1975–77, DHMECN 2548, DHMECN 3831–32, DHMECN 9442–47; Sendero Ñalacapac, DHMECN 10867. Hyloscirtus sp.2 (13): Ecuador: Carchi (12): Cabeceras del Río Baboso, DHMECN 1000–07; El Baboso, cascada DHMECN 14910; El Baboso, zona alta DHMECN14897, 14901, El Goaltal, DHMECN 1045; Esmeraldas (1): Alto Tambo-Río Negro, DHMECN 3281. |
| Species | SVL (males) in mm |
SVL (females) in mm | Snout shape | Tip of snout | Anillus tympanic | Supranal fold | Calcars |
|---|---|---|---|---|---|---|---|
| Hyloscirtus concientia sp. nov | 29.6–33.3 (14) mean = 31 | 34.7–40 (3) mean = 37.3 | Snout subacuminate in dorsal view and rounded in lateral view | Rounded | Conspicuos | Present | Absent |
| Hyloscirtus mashpi | 28.7–33.8 (14) mean = 31.5 | 37–38.5 (2) mean = 37.8 | Snout rounded in dorsal view and lateral view | Truncate | Inconspicuos | Absent | Absent |
| Hyloscirtus alytolylax | 32.1–37.0 (13) mean = 34.85 | 37.2–43.9 (15) mean = 40.3 | Snout rounded in dorsal view and lateral view | Truncate | Conspicuos | Present | Absent |
| Hyloscirtus palmeri | 34.9–42.6 (15) mean = 39.6 | 35.7–50.0 (7) mean = 44.4 | Snout rounded in dorsal view and truncate in lateral view | Truncate | Conspicuos | Present | Present |
| Hyloscirtus simmonsi | 35–37.8 (12) mean = 39.7 | 36.6 (1) | Snout subacuminate in dorsal view and inclined posteroventrally from tipo to margen of jaw in lateral view | Truncate | Barely evident | Absent | Absent |
| Species | Dorsal coloration pattern in live | Iridophoros | Melanophoros | Canthal stripe cream | Supraocular stripe cream | Tympanic stripe cream | Sourse |
|---|---|---|---|---|---|---|---|
| Hyloscirtus concientia sp. nov | Dorsum usually pale yellowish-green, usually with a thin brown mid-dorsal stripe | With individual iridophores sometimes packed on top of melanophores but never forming dots | Melanophores smaller and more densely packed | Present | Present | Present | This work |
| Hyloscirtus mashpi | Pale greenish yellow with a brown dorsal midline | Iridophores forming dots, scarcely distributed across dorsum | Mid-dorsal stripe formed by melanophores larger and less dense | Present | Present | Present | Guayasamin et al. (2015), this work |
| Hyloscirtus alytolylax | Greenish brown to pale green | Iridophores forming abundant dots | Absent | Present | Present | Present | Duellman, 1972, this work |
| Hyloscirtus palmeri | Dark green back with few irregular white or cream spots | Absent | When present, evenly distributed on the back | Absent | Absent | Absent | Duellman, 1972, this work |
| Hyloscirtus simmonsi | Dull green with minute brown flecks | Absent | Thickly covered by melanophores | Absent | Absent | Absent | Duellman, 1989, this work |