Complete chloroplast genome sequences of five Bruguiera species (Rhizophoraceae): comparative analysis and phylogenetic relationships
- Published
- Accepted
- Received
- Academic Editor
- Victoria Sosa
- Subject Areas
- Genetics, Genomics, Molecular Biology, Plant Science
- Keywords
- Bruguiera, Mangrove, Chloroplast genome, Rhizophoraceae, Comparative analysis, Phylogenetic relationships
- Copyright
- © 2021 Ruang-areerate et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Complete chloroplast genome sequences of five Bruguiera species (Rhizophoraceae): comparative analysis and phylogenetic relationships. PeerJ 9:e12268 https://doi.org/10.7717/peerj.12268
Abstract
Bruguiera is a genus of true mangroves that are mostly distributed in the Indo-West Pacific region. However, the number of published whole chloroplast genome sequences of Bruguiera species are limited. Here, the complete chloroplast sequences of five Bruguiera species were sequenced and assembled using Illumina data. The chloroplast genomes of B. gymnorhiza, B. hainesii, B. cylindrica, B. parviflora and B. sexangula were assembled into 161,195, 164,295, 164,297, 163,228 and 164,170 bp, respectively. All chloroplast genomes contain 37 tRNA and eight rRNA genes, with either 84 or 85 protein-coding genes. A comparative analysis of these genomes revealed high similarity in gene structure, gene order and boundary position of the LSC, SSC and two IR regions. Interestingly, B. gymnorhiza lost a rpl32 gene in the SSC region. In addition, a ndhF gene in B. parviflora straddles both the SSC and IRB boundary regions. These genes reveal differences in chloroplast evolution among Bruguiera species. Repeats and SSRs in the chloroplast genome sequences were found to be highly conserved between B. cylindrica and B. hainesii as well as B. gymnorhiza and B. sexangula indicating close genetic relationships based on maternal inheritance. Notably, B. hainesii, which is considered a hybrid between B. gymnorhiza and B. cylindrica, appears to have inherited the chloroplast from B. cylindrica. Investigating the effects of selection events on shared protein-coding genes showed a positive selection in rps7 and rpl36 genes in all species compared to land-plant species. A phylogenetic analysis, based on 59 conserved chloroplast protein-coding genes, showed strong support that all Bruguiera species are in the clade Rhizophoraceae. This study provides valuable genetic information for the study of evolutionary relationships and population genetics in Bruguiera and other mangrove species.
Introduction
Mangroves are plants that live in coastal regions across tropical and sub-tropical regions. They play an important role in ecology by providing habitats and nurseries for many marine organisms and protecting coastlines from erosion. There are approximately 70 mangrove species belonging to 16 families (Kathiresan & Bingham, 2001). Rhizophoraceae is a true mangrove family consisting of ~18 species in four genera, which are Bruguiera, Ceriops, Kandelia and Rhizophora (Tomlinson, Primack & Bunt, 1979).
Bruguiera is a dominant and economically important mangrove genus. It produces wood that is used for fuel and house construction, and produces several useful metabolites such as diterpenes and triterpenes (Dahdouh-Guebas et al., 2000; Han et al., 2004; Nebula, Harisankar & Chandramohanakumar, 2013). This genus comprises six species, namely B. cylindrica, B. exaristata, B. gymnorhiza, B. hainesii, B. parviflora and B. sexangula as well as their hybrids such as B. x rhynchopetala and B. x dungarra (Duke & Ge, 2011; Duke & Kudo, 2018). Among them, B. gymnorhiza is the most widespread mangrove plant, while B. hainesii is a critically endangered mangrove plant (FAO, 2007; Duke & Ge, 2011). Most Bruguiera species are distributed in the Indo-West Pacific region, except B. exaristata that is mostly distributed in Northern Australia and Southern New Guinea (FAO, 2007; Duke & Ge, 2011; Urashi et al., 2013). Based on morphological characters, the genus Bruguiera is separated into two major groups (Hou, 1958; Sheue, Yong & Yang, 2005; Duke & Ge, 2011). One group (B. gymnorhiza, B. exaristata and B. sexangula) has large leaves and large solitary-flowered inflorescences, while the other group (B. cylindrica, B. hainesii and B. parviflora) has small leaves, small multiple-flowered inflorescences. In mangrove forests, the distributions of Bruguiera species overlap each other and it can be difficult to identify species based on morphological characters (Abeysinghe et al., 1999; Duke & Ge, 2011). Consequently, molecular markers such as random amplified polymorphic DNA (RAPD) and simple sequence repeats (SSRs) based on nuclear and chloroplast sequences have been developed for species identification in Bruguiera (Abeysinghe et al., 1999; Islam et al., 2008).
Chloroplasts are semi-autonomous organelles in algae, cyanobacteria and plants that are responsible for photosynthesis. Chloroplast genomes have been used to study plant species identification, population genetics, genome evolution and phylogenetics (Shi et al., 2005; Dong et al., 2012; Saddhe, Jamdade & Kumar, 2016; Bi et al., 2018; Wu et al., 2019). For example, DNA barcoding based on chloroplast markers such as matK, rbcL and some intergenic spacer regions (i.e., trnL-trnF and trnH-psbA) is an effective tool to identify mangrove species and their evolutionary relationships (Shi et al., 2005; Saddhe, Jamdade & Kumar, 2016; Wu et al., 2019). In most land plants, chloroplast genomes are inherited maternally. The chloroplast structure is usually a small circular quadripartite DNA consisting of one large single copy (LSC) region, one small single copy (SSC) region and two inverted repeats (IRs), which separate the LSC and SSC (Mower & Vickrey, 2018). Some plant lineages have lost one copy of the IR, such as papilionoid legumes (Palmer & Stein, 1986; Choi, Jansen & Ruhlman, 2019). Over the past decade, next generation sequencing technologies have been used to generate hundreds of complete chloroplast genomes (Tangphatsornruang et al., 2010; Tangphatsornruang et al., 2011; Uthaipaisanwong et al., 2012). Recently, a number of complete chloroplast genomes of mangroves in the family Rhizophoraceae were reported (Li et al., 2019; Yang et al., 2019; Zhang, Zhong & Yuan, 2019; Shi et al., 2020; Li et al., 2021). These mangrove chloroplast genomes are 160–164 kb in length and consist of all four regions with LSC; ~91–93 kb, SSC; ~16–19 kb and IRs; ~26–27 kb. They contain 128–131 genes and have a highly conserved organization and structure. To date, only the chloroplast genomes of B. sexangula and B. gymnorhiza are available (Shi et al., 2020; Li et al., 2021). Thus, the chloroplast genome sequences in the genus Bruguiera are limited.
In this study, the chloroplast genomes of five Bruguiera species (B. cylindrica, B. gymnorhiza, B. hainesii, B. parviflora and B. sexangula) in the family Rhizophoraceae were sequenced, assembled and compared to reveal their evolutionary relationship. These chloroplast genomes will provide valuable genetic information and better understanding of evolutionary relationships among Bruguiera species.
Materials & Methods
DNA extraction and sequencing
Fresh leaves in five Bruguiera species (B. cylindrica, B. gymnorhiza, B. hainesii, B. parviflora, and B. sexangula) were collected from Ranong province in Thailand (Table S1) and stored in liquid nitrogen. DNA samples were extracted using a standard CTAB (Cetyl Trimethyl Ammonium Bromide) method (Doyle & Doyle, 1987). Then the genomic DNA was sequenced using an Illumina Hiseq2000 platform according to Illumina protocols.
Chloroplast genome assembly and annotation
Based on a reference genome-based strategy, the Illumina paired-end reads and B. sexangula (the accession CNA0003536 in the project-ID CNP0000567 in CNSA (https://db.cngb.org/cnsa) (Shi et al., 2020)) were used for assembling the chloroplast genomes of the five Bruguiera species using GetOrganelle (Jin et al., 2020).
The chloroplast genome sequences were annotated using GeSeq (Tillich et al., 2017). The start-stop loci and intron-exon junctions of coding genes were checked manually based on reported chloroplast genes of other land-plant species. In addition to the coding genes, transfer RNAs (tRNAs) were identified using ARAGORN v1.2.36 (Laslett & Canback, 2004) in the GeSeq software. The circular structures of the chloroplast genomes were visualized using OGDRAW v1.3.1 (Greiner, Lehwark & Bock, 2019). The sequences of the five chloroplast genomes were deposited in GenBank (accession number MW836110–MW836114).
In addition, raw reads in each species were mapped to the rpl32 sequence using BWA (Li & Durbin, 2009) and one primer pair was designed to amplify the ndhF-rpl32 region (Table S2) (Shaw et al., 2007) in order to examine evidence for the rpl32 gene loss in the assembled chloroplast-genome sequence of B. gymnorhiza.
Comparative genome analysis
The comparison of whole chloroplast genomes among the Bruguiera species and two close non-mangrove species, Pellacalyx yunnanensis (MN106253) of Rhizophoraceae and Erythroxylum novogranatense (NC_0306061) of Erythroxylaceae, were analyzed to investigate sequence divergence using mVISTA with the Shuffe-LAGAN mode (Frazer et al., 2004). The previously reported chloroplast genome of B. sexangula was used as a reference for comparison (Shi et al., 2020). Furthermore, the junctions and borders of the IR regions were illustrated using IRscope (Amiryousefi, Hyvönen & Poczai, 2018).
Repeat and SSR identification
Repeat sequences with length ≥50 bp were identified using REPuter (Kurtz et al., 2001). Furthermore, SSRs in the chloroplast genome sequences of the five Bruguiera species and three previously reported mangrove species, Kandelia obavata (MH277332), Rhizophora x lamarckii (NC_046517) and Rhizophora stylosa (MK070169), in the family Rhizophoraceae were identified using MISA (Thiel et al., 2003). Minimum repeat number requirements were set to 10 for mono-, five for di-, four for tri-, and three for tetra-, penta- and hexa-nucleotide repeats (Shi et al., 2020). The distance for compound SSRs was ≤100 bp (default).
Four polymorphic SSR loci were selected to examine polymorphism in different Bruguiera species. SSR primer pairs were designed for the four SSR loci and the resulting PCR product sizes were determined for each species (Table S3).
Phylogenetic analysis
To validate the phylogenetic relationships of the Bruguiera species based on plastid genes, 9 representative families (Rhizophoraceae, Erythroxylaceae, Ctenolophonaceae, Violaceae, Salicaceae, Passifloraceae, Euphorbiaceae, Malpighiaceae and Clusiaceae) were selected from the order Malpighiales to construct phylogenetic tree. A total of 59 conserved chloroplast coding-genes in 40 species including the five species in this study, three other mangrove species, 31 land plant species and one outgroup species in the order Oxalidales were retrieved from NCBI and used to construct a phylogenetic tree (Table S4). These genes are atpA, atpB, atpE, atpF, atpH, atpI, ccsA, cemA, clpP, matK, ndhA, ndhD, ndhE, ndhG, ndhH, ndhI, ndhJ, ndhK, petA, petD, petG, petL, petN, psaA, psaB, psaC, psaI, psaJ, psbA, psbC, psbD, psbF, psbH, psbJ, psbL, psbM, psbN, psbT, rbcL, rpl2, rpl14, rpl16, rpl33, rpl36, rpoA, rpoB, rpoC1, rpoC2, rps2, rps3, rps4, rps8, rps11, rps12, rps14, rps15, rps18, rps19 and ycf3 (Table S4). Each coding-gene sequence was aligned using MUSCLE with default parameters in MEGA X (Kumar et al., 2018), and the aligned sequences in all genes were concatenated for each species. The best-fit model of plastid DNA substitution (GTR + I + G) was identified using the find best DNA/protein model tool in MEGA X. Based on the aligned sequences, a maximum likelihood (ML) phylogenetic tree was preformed using RAxML version 8.2.10 (Stamatakis, 2014) with GTRGAMMAI (GTR + I + G) model and 1,000 bootstrap values. Averrhoa carambola (NC_033350) in Oxalidales was used to be an outgroup species in the phylogenetic tree (Wurdack & Davis, 2009). The phylogenetic trees were visualized using FigTree v1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/). In addition, chloroplast rpl32 gene loss in B. gymnorhiza and other plant species in Malpighiales and Oxalidales (Alqahtani & Jansen, 2021) were plotted onto the their clades of the phylogenetic tree.
Gene selective pressure analysis of Bruguiera chloroplasts
A total 74 shared plastid protein-coding genes in the five Bruguiera species and four terrestrial plant species, Erythroxylum novogranatense (NC_030601), Ctenolophon englerianus (NC_049158), Averrhoa carambola (NC_033350) and Vitis rotundifolia (NC_023790) (Table S5), were used to investigate selection pressures. Pairwise sequence alignments for each gene between the Bruguiera species and the terrestrial plant species based on the background of different evolutionary clades, including Erythroxylaceae (E. novogranatense), Ctenolophonaceae (C. englerianus), Oxalidales (A. carambola), and Rosids (Vitis rotundifolia) (Han et al., 2020) were generated using MUSCLE with default setting in MEGA X (Edgar, 2004; Kumar et al., 2018). Gaps between compared sequences were removed before further analysis. Nonsynonymous (Ka) and synonymous (Ks) substitution rates (Ka/Ks) of each gene were calculated using KaKs Calculator version 2.0 (Wang et al., 2010), with its model-averaging method.
Results
Chloroplast genome features
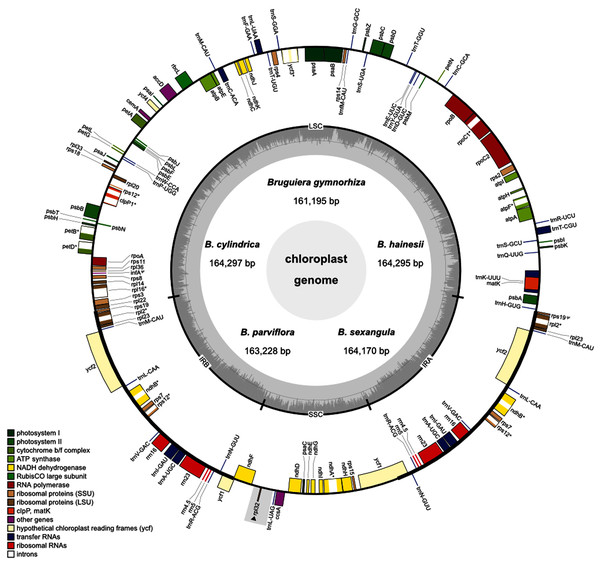
Five Bruguiera species (B. gymnorhiza, B. hainesii, B. cylindrica, B. parviflora and B. sexangula) were sequenced to generate 743,609,903–800,931,061 raw reads per species (150 bp in length) using Illumina Hiseq2000 data (Table 1). A total of 4,866,585–7,635,727 pair-end reads for each species mapped to the reference chloroplast genome of B. sexangula (Shi et al., 2020) and were used to assemble their chloroplast genome sequences. The read coverage was estimated at over 300× for each species. The size of the assembled chloroplast genomes ranged from 161,195 (B. gymnorhiza) to 164,297 (B. cylindrica) bp (Table 1; Fig. 1). Notably, the difference in genome length of B. cylindrica (164,297 bp) and B. hainesii (164,295 bp) was just 2 bp. All chloroplast genome sequences formed the typical quadripartite circular structure with the four regions including one large single copy (LSC), one small single copy (SSC) and two inverted repeat regions (IRA and IRB). The length of the LSC (91,154–91,738 bp) and IR (26,299–26,393 bp) regions was highly similar in all species, whereas the size of the SSC region had the highest variation and ranged from 17,053 (B. gymnorhiza) to 19,956 bp (B. sexangula) (Table 1). The average GC content in all chloroplast genomes was approximately 35%. The GC content in the IR regions (~42%) was higher than in the LSC (~33%) and SSC (~29%) regions.
Figure 1: Circular map of the five Brugueira chloroplast genomes.
The inner circle shows the GC content of the chloroplast genome. LSC, SSC, and IRs (IRA and IRB) are large single copy, small single copy, and inverted repeats, respectively. Genes based on different functional groups are shown in different colors. Grey rectangle indicates a loss region of B. gymnorhiza.| Species | B. gymnorhiza | B. cylindrica | B. hainesii | B. parviflora | B. sexangula |
|---|---|---|---|---|---|
| Raw reads | 743,609,903 | 791,571,475 | 755,597,881 | 781,228,900 | 800,931,061 |
| Reads for chloroplast analysis | 7,077,428 | 5,257,482 | 4,866,585 | 7,148,202 | 7,635,727 |
| Base-coverage (x) | 348 | 372 | 366 | 377 | 364 |
| Genome size (bp) | 161,195 | 164,297 | 164,295 | 163,228 | 164,170 |
| LSC (bp) | 91,404 | 91,738 | 91,736 | 91,154 | 91,428 |
| SSC (bp) | 17,053 | 19,839 | 19,839 | 19,476 | 19,956 |
| IR (bp) | 26,369 | 26,360 | 26,360 | 26,299 | 26,393 |
| LSC GC content (%) | 32.77 | 32.69 | 32.69 | 32.86 | 32.77 |
| SSC GC content (%) | 29.85 | 28.38 | 28.38 | 28.96 | 28.31 |
| IR GC content (%) | 42.19 | 42.20 | 42.20 | 42.26 | 42.19 |
| Genome GC content (%) | 35.54 | 35.22 | 35.22 | 35.43 | 35.25 |
| No. of genes | 129 | 130 | 130 | 130 | 130 |
| No. of protein coding genes | 82 | 83 | 83 | 83 | 83 |
| No. of rRNAs | 8 | 8 | 8 | 8 | 8 |
| No. of tRNAs | 37 | 37 | 37 | 37 | 37 |
| No. of duplicated genes | 17 | 17 | 17 | 17 | 17 |
| Pseudogenes | 2 | 2 | 2 | 2 | 2 |
| Gene loss | rpl32 | – | – | – | – |
A total of 129 (B. gymnorhiza)–130 (B. hainesii, B. cylindrica, B. parviflora and B. sexangula) genes including 84–85 protein-coding genes, 37 transfer (tRNA) genes and eight ribosomal RNA (rRNA) genes were identified (Fig. 1, Tables 1 and 2). Of these, 19 genes (ndhB, rpl2, rpl23, rps7, rps12, rps19, ycf1, ycf2, rrn4.5, rrn5, rrn16, rrn23, trnA-UGC, trnI-GAU, trnL-CAA, trnM-CAU, trnN-GUU, trnR-ACG, and trnV-GAC) are duplicated in the IR regions (Fig. 1 and Table 2). All photosynthesis genes, including photosystems I (five genes) and II (15 genes), NADH dehydrogenase (11 genes), cytochrome b/f complex (six genes), ATP synthase (six genes) and rubisco large subunit (rbcL), were found in all Bruguiera chloroplast genomes. Several large and small subunits of ribosomal proteins (rpl and rps) were also identified (Table 2). Interestingly, a rpl32 gene was lost only in B. gymnorhiza (Tables 1 and 2). The loss was detected from low read coverage in this region of B. gymnorhiza (Table S6) and confirmed by lack of a PCR product from the ndhF-rpl32 region in B. gymnorhiza compared with other Bruguiera species (Fig. S1). Furthermore, other metabolic genes including maturase (matK), acetyl-CoA-carboxylate (accD), chloroplast envelope membrane protein (cemA), ATP-dependent protease (clpP), cytochrome c synthesis (ccsA) and conserved open reading frames (four genes) were found in all chloroplast genomes. There are two pseudogenes (ycf1 and rps19) in the chloroplast genomes (Table 2). Among all identified genes, eight protein-coding genes (atpF, ndhA, ndhB, petB, petD, rpl2, rpl16 and rpoC1) contain two exons and two protein-coding genes (rps12 and ycf3) contain three exons (Tables 2 and S7). The introns in these genes in the Bruguiera species are highly conserved (Table S7). Several tRNA genes also contain introns such as trnA-UGC, trnC-ACA, trnI-GAU, trnK-UUU, trnL-UAA and trnT-CGU. The trnK-UUU intron in these chloroplast genomes encodes the protein-coding gene of matK (Fig. 1).
| Category | Group of genes | Gene name |
|---|---|---|
| Photosynthesis | Subunits of Photosystem I | psaA, B, C, I, J |
| Subunits of Photosystem II | psbA, B, C, D, E, F, H, I, J, K, L, M, N, T, Z | |
| Subunits of NADH dehydrogenes | ndhA*, B*(×2), C, D, E, F, G, H, I, J, K | |
| Cytochrom b6/f complex | petA, B*, D*, G, L, N | |
| ATP synthase | atpA, B, E, F*, H, I | |
| Rubisco | rbcL | |
| Self-replication | Large subunit of ribosomal proteins | rpl2*(×2), 14, 16*, 20, 22, 23(×2), 32a, 33, 36 |
| Small subunit of ribosomal proteins | rps2, 3, 4, 7(×2), 8, 11, 12**(×2), 14, 15, 18, 19 | |
| DNA dependent RNA polymerase | rpoA, B, C1*, C2 | |
| rRNA genes | rrn4.5(×2), 5(×2), 16(×2), 23*(×2) | |
| tRNA genes | trnA-UGC*(×2), trnC-ACA*, trnC-GCA, trnD-GUC, | |
| trnE-UUC, trnF-GAA, trnfM-CAU, trnG-GCC, | ||
| trnH-GUG, trnI-GAU*(×2), trnK-UUU*, | ||
| trnL-CAA(×2), trnL-UAA*, trnL-UAG, trnM-CAU(×3), | ||
| trnN-GUU(×2), trnP-UGG, trnQ-UUG, | ||
| trnR-ACG(×2), trnR-UCU, trnS-GCU, | ||
| trnS-GGA, trnS-UGA, trnT-CGU*, trnT-GGU | ||
| trnT-UGU, trnV-GAC(×2), trnW-CCA, trnY-GUA | ||
| Other genes | Maturase | matK |
| Subunit Acetyl-CoA-Carboxylate | accD | |
| Envelop membrane protein | cemA | |
| Proteaese | clpP** | |
| C-type cytochrome synthesis gene | ccsA | |
| Unknows | Conserved Open reading frames | ycf1, 2(×2), 3**, 4 |
| pseudogene | ycf1, rps19 |
Chloroplast genome comparison
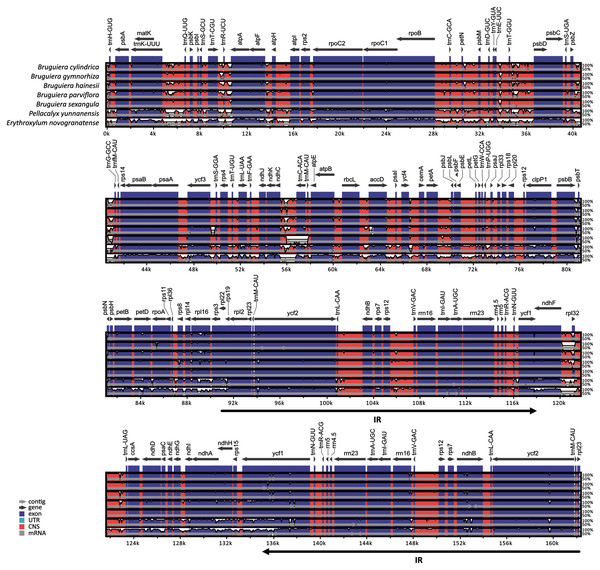
Comparison of chloroplast genomes in the five Bruguiera species and two closely related non-mangrove species (P. yunnanensis and E. novogranatense) revealed similar genome organization and gene order with some sequence variation (Fig. 2). Gene orientation was assessed by synteny analysis among the species, revealing conserved gene structure in the chloroplast genomes. The coding regions were more conserved than the intergenic regions. Both the coding and intergenic regions were highly similar in all Bruguiera species compared to the two non-mangrove species. The LSC and SSC regions had higher divergence than the IR regions indicating that the IR regions are highly conserved. A comparison between the two B. sexangula chloroplast genomes, previously reported (Shi et al., 2020) and the one generated in this study (Fig. 2), showed several small variations, including the difference of mononucleotide repeats, short repeats and indels. Furthermore, several poorly conserved regions located in the LSC (such as ~40.2–41.2 kb and ~55.5–56.8 kb) and SSC (such as ~120.0–121.3 kb) regions were found between the Bruguiera species and E. novogranatense. Some intergenic regions contained more variation among the species such as regions between trnK-trnQ, trnT-trnR, trnR-atpA, trnT-psbD, psbZ-trnG, ndhC-trnC and ndhF-rpl32.
Figure 2: Alignment of the chloroplast genomes of five Bruguiera and two close non-mangrove species.
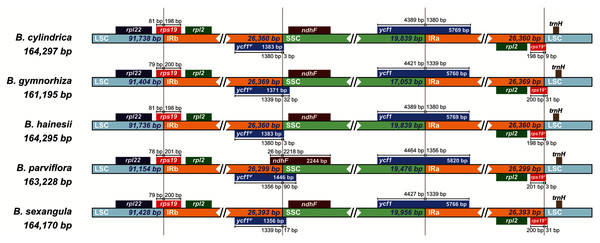
The sequence identity was plotted among the chloroplast genomes, with B. sexangula (CNA0003536) as a reference using the mVista software. Grey and black arrows indicate genes and IR regions, respectively. Blue sections represent coding regions, and pink sections represent conserved non-coding sequences.Moreover, the chloroplast boundary structures of the LSC, SSC and IRs were compared among the five species (Fig. 3). In all species, the ycf1 gene and the ycf1 pseudogene were located at the boundary of SSC/IRA and SSC/IRB, respectively, while the rps19 gene and the rps19 pseudogene were located at the boundary of LSC/IRB and LSC/IRA, respectively. The length of the ycf1 and rps19 pseudogenes varied from 1,371 bp (B. gymnorhiza) to 1,446 bp (B. parviflora) and from 204 bp (B. parviflora) to 231 bp (B. gymnorhiza and B. sexangula), respectively. The LSC/IR and SSC/IR borders of B. cylindrica and B. hainesii were more similar than the LSC/IR and SSC/IR borders of the other species. For example, the ycf1 pseudogene is 3 bp away from the SSC/IRB border in both B. cylindrica and B. hainesii, whereas it is 32, 90 and 17 bp in B. gymnorhiza, B. parviflora and B. sexangula, respectively. The rps19 pseudogene is 9 bp away from the LSC/IRA border in both B. cylindrica and B. hainesii, while it is 31, 3 and 31 bp in B. gymnorhiza, B. parviflora and B. sexangula, respectively. Additionally, the ndhF gene of B. parviflora straddles both the IRB and SSC boundary regions, with 26 bp of the IRB region and 2,218 bp of the SSC region, whereas the gene is located within the SSC region in all other species. While the sequence here is essentially the same in all species, B. parviflora has a variant that removes a stop codon causing ycf1ψ to overlap ndhF in the SSC/IRB boundary region.
Figure 3: Comparisons of the borders of LSC, SSC, and IR regions among the five Bruguiera chloroplast genomes.
Repeat and SSR contents
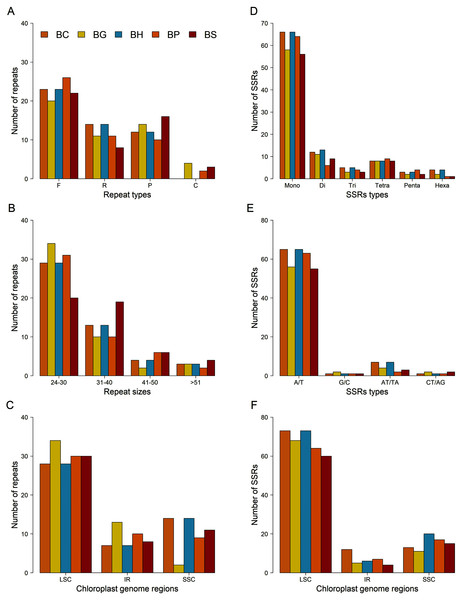
A total of 49 repeats, consisting of forward, reverse, palindromic and complement repeats, were identified in the chloroplast genome of each Bruguiera species (Figs. 4A–4C and Table S8). Among them, the most common repeat type was forward repeat (Fig. 4A). The repeat patterns from B. cylindrica and B. hainesii were same. Most repeats ranged in length from 24 to 30 bp (Fig. 4B) and were in the LSC region (Fig. 4C).
Figure 4: Comparison of repeats and microsatellites (SSRs) among the five Bruguiera chloroplast genomes.
(A) Number of repeats based on types; (B) Number of repeats based on sizes; (C) Distribution of repeats in chloroplast regions; (D) Number of SSRs based on types; (E) Number of SSRs based on mononucleotides and dinucleotides; (F) Distribution of SSRs in chloroplast regions. BC, BG, BH, BP and BS represent B. cylindrica, B. gymnorhiza, B. hainesii; B. parviflora and B. sexangula, respectively.SSR loci in the chloroplast genomes of the five Bruguiera species and three previously reported mangrove species in the family Rhizophoraceae were identified (Figs. 4D–4F; Tables 3 and S9). The number of SSR loci (simple, compound, and interrupted SSRs) varied among the species, ranging from 164 in Kandelia obavata to 197 in B. hainesii (Table 3). The number of SSR in B. hainesii (197) was higher than B. cylindrica (186) because of a variant that increased the repeat count of an SSR to reach the minimum threshold for inclusion. Among simple SSR, the number of mononucleotide repeats accounted for ~56–68% of all loci, of which A/T repeats were the most frequent type (Figs. 4D–4E). Most of the SSR loci were in the LSC region (Fig. 4F). In addition to simple SSR loci, there were numerous compound and interrupted SSR loci (more than two different repeat types present within ≤100 bases) (Tables 3, S9 and S10). Most Bruguiera species have more compound and interrupted SSR loci than other mangrove species in the family Rhizophoraceae. Each SSR loci were present in many Bruguiera species, with the most sharing between B. cylindrica and B. hainesii, and between B. gymnorhiza and B. sexangula (Table S10; Fig. S2). Two compound SSRs with 64 and 98 bp located in noncoding and intron-ndhA regions, respectively, were found in all Bruguiera chloroplast genomes. Notably, SSR loci of the Bruguiera chloroplast genomes were unique compared with the other mangrove species (K. obavata, R. stylosa and R. x lamarckii) in the family Rhizophoraceae.
| Species | SSR type | Total number | The number of SSRs for compound formation | |||||
|---|---|---|---|---|---|---|---|---|
| Mono- | Di- | Tri- | Tetra- | Penta- | Hexa- | |||
| BH | 119 | 31 | 16 | 16 | 9 | 6 | 197 | 61 |
| BC | 119 | 30 | 16 | 16 | 9 | 6 | 196 | 61 |
| BG | 102 | 26 | 14 | 18 | 4 | 2 | 166 | 51 |
| BP | 109 | 31 | 17 | 23 | 8 | 1 | 189 | 64 |
| BS | 109 | 36 | 16 | 19 | 5 | 1 | 186 | 70 |
| BSa | 106 | 31 | 14 | 18 | 5 | 1 | 175 | 58 |
| KO | 92 | 29 | 17 | 18 | 8 | 0 | 164 | 50 |
| RL | 117 | 22 | 13 | 10 | 9 | 2 | 173 | 52 |
| RS | 117 | 22 | 13 | 10 | 9 | 2 | 173 | 52 |
Notes:
BC, B. cylindrica (MW836110); BG, B. gymnorhiza (MW836111); BH, B. hainesii (MW836112); BP, B. parviflora (MW836113); BS, B. sexangula (MW836114); BSa, B. sexangula (CNA0003536); KO, Kandelia obavata (MH277332); RL, Rhizophora x lamarckii (NC_046517); RS, Rhizophora stylosa (MK070169) and PY, Pellacalyx yunnanensis (MN106253).
Phylogenetic relationships
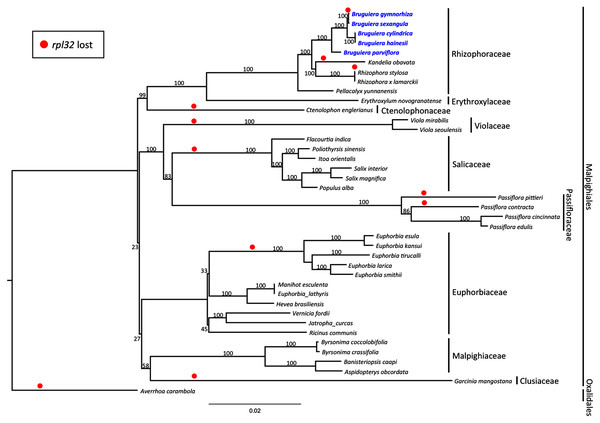
Maximum likelihood (ML) phylogenetic analysis was performed based on 59 highly conserved chloroplast coding genes in 40 species including 39 plant species in Malpighiales and one outgroup species in Oxalidales (Fig. 5). The genetically distinct clades were classified into the lineages of nine families in Malpighiales consisting of nine Rhizophoraceae members, one Erythroxylaceae member, one Ctenolophonaceae member, two Violaceae members, six Salicaceae members, four Passifloraceae members, 11 Euphorbiaceae members, four Malpighiaceae members and one Clusiaceae member. The phylogenetic tree shows that all Bruguiera species were in the lineage Malpighiales and grouped under the clade Rhizophoraceae with a 100% bootstrap support. B. gymnorhiza was placed closely with B. sexangula, while B. cylindrica and B. hainesii were grouped together. B. parviflora is a sister species of the other four Bruguiera species. Loss of the rpl32 gene was observed in several land-plant species (Fig. 5), such as Ctenolophon engierianus, Viola species, species in Salicaceae and Euphorbia species, and mangrove species such as B. gymnorhiza, K. obavata and R. stylosa.
Figure 5: Molecular phylogenetic tree of the five Bruguiera species, three other mangrove species and thirty-two terrestrial plant species based on 59 conserved chloroplast coding genes.
The maximum likelihood tree was constructed using RAxML program with the GTR + G + I nucleotide model. Numbers above nodes are bootstrap values with 1,000 replicates. Averrhoa carambola (NC_033350) was set as the outgroups. Loss of the rpl32 gene is indicated by red circle.Selective pressure genes in Bruguiera mangrove species
To investigate gene selection pressures, the nonsynonymous and synonymous substitution (Ka/Ks) ratio was calculated by comparing 74 conserved chloroplast gene sequences of each Bruguiera species to assumed ancestors, which were E. novogranatense (Erythroxylaceae), C. englerianus (Ctenolophonaceae), A. carambola (Oxalidales) and V. rotundifolia (Rosids) (Table S11). Most Ka/Ks values of compared pairs were less than 1.0. There were two genes, rps7 and rpl36, in which the Ka/Ks ratios were higher than one compared with V. rotundifolia (Rosids) and E. novogranatense (Erythroxylaceae), respectively suggesting positive selection during their evolution. The Ka/Ks ratios of the rps7 gene between B. cylindrica, B. gymnorhiza, B. hainesii, B. parviflora and B. sexangula compared with V. rotundifolia were 1.58, 1.53, 1.58, 1.69 and 1.53, respectively. The Ka/Ks ratios of the rpl36 gene between B. cylindrica, B. gymnorhiza, B. hainesii, B. parviflora and B. sexangula compared with E. novogranatense were 1.10, 1.10, 1.10, 0.94 and 1.10, respectively. The Ka/Ks ratios for the rps7 gene were close to 1 when compared with E. novogranatense (Ka/Ks average of the five Bruguiera species = 0.91), C. englerianus (0.94), and A. carambola (0.97) (Table S11) revealing neutral selection within the lineages. The rps7 gene of the Bruguiera species compared with V. rotundifolia contained 13 variation sites including 11 non-synonymous and two synonymous sites (Table 4). Five of the eleven non-synonymous sites (amino acid position: 21, 45, 105, 121, and 122) were unique to the Bruguiera species revealing specific nucleotide mutations during their evolution. Similar to the rps7 gene, the rpl36 gene of the Bruguiera species compared with E. novogranatense contained eight variation sites including six non-synonymous and two synonymous sites (Table 4). Three out of the six non-synonymous sites (amino acid position: five and 24 (two variation sites for one codon change)) were specific to the Bruguiera species.
| Gene | Nucleotide position | Nucleotide base | Amino acid position | Amino acid reference | Amino acid change | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| V. rotundifolia | A. carambola | C. emglerianus | E. novogranatense | B. cylindrica | B. gymnorhiza | B. hainesii | B. parviflora | B. sexangula | |||||
| rps7 | 62 | G | G | G | G | A | G | A | G | G | 21 | R | Q |
| 99 | C | C | C | T | T | T | T | T | T | 33 | H | H | |
| 133 | C | C | C | C | G | G | G | G | G | 45 | R | G | |
| 136 | T | G | G | G | G | G | G | G | G | 46 | S | A | |
| 139 | G | A | A | A | A | A | A | A | A | 47 | V | M | |
| 167 | C | C | C | A | A | A | A | A | A | 56 | T | K | |
| 173 | C | C | C | G | G | G | G | T | G | 58 | P | R/L | |
| 187 | C | C | C | T | T | T | T | T | T | 63 | R | C | |
| 314 | C | C | C | C | T | T | T | T | T | 105 | S | F | |
| 326 | C | C | C | T | T | T | T | T | T | 109 | P | L | |
| 363 | A | A | A | A | T | T | T | T | T | 121 | L | F | |
| 364 | G | G | G | G | T | T | T | T | T | 122 | V | L | |
| 372 | T | T | T | C | C | C | C | C | C | 124 | A | A | |
| rpl36 | 13 | G | G | G | G | A | A | A | G | A | 5 | A | T |
| 19 | G | G | A | A | G | G | G | G | G | 7 | I | V | |
| 44 | G | G | G | G | A | A | A | G | A | 15 | R | Q | |
| 55 | A | A | A | A | C | C | C | C | C | 19 | R | R | |
| 63 | A | A | A | A | A | A | A | G | A | 21 | G | G | |
| 70 | A | A | A | A | T | T | T | T | T | 24 | I | F | |
| 72 | A | A | A | A | C | C | C | C | C | 24 | I | F | |
| 82 | C | T | C | C | T | T | T | T | T | 28 | P | S | |
Discussion
In the present study, we have determined the complete chloroplast genome sequence of five Bruguiera species using Illumina sequence data. The chloroplast genomes have highly conserved structure and gene content, as observed in most land plants (Daniell et al., 2016). The size of the chloroplast genomes ranged from 161–164 kb, concordant with other mangrove species (160–164 kb) in the family Rhizophoraceae (Li et al., 2019; Yang et al., 2019; Zhang, Zhong & Yuan, 2019; Shi et al., 2020; Li et al., 2021). The genomes consisted of four regions (LSC, SSC and two IRs) and the IR regions were highly conserved. Interestingly, B. gymnorhiza (161,195 bp), which is a widespread species in the Indo-West Pacific region, had a smaller chloroplast size than the other Bruguiera species (163,228–164,297 bp) because of a shorter SSC region compared with the others.
In all species, the chloroplast genomes contained the same number of tRNA (37) genes, rRNA (8) genes and pseudogenes (2), whereas the number of protein-coding genes (84–85) was slightly different. The rpl32 gene in the SSC region was lost in B. gymnorhiza, which was validated by PCR amplification of the ndhF-rpl32 region (Fig. S1). Loss of the rpl32 gene was found in at least 73 species in eudicots, occurring through multiple independent loss events, such as K. obovata (mangrove) and Populus species (Ueda et al., 2007; Yang et al., 2019; Mohanta et al., 2020). The transfer of the rpl32 gene from chloroplast to the nuclear genomes occurred several times across angiosperms (Ueda et al., 2007; Yang et al., 2019; Mohanta et al., 2020). In this study, we found that this gene was likely transferred to the nuclear genome of B. gymnorhiza due to low read coverage (~100×) compared with the other Bruguiera species (~8,000×) (Table S6). These results suggest that the rpl32 gene was independently lost in chloroplasts of mangrove species.
The sequence and content of the IR regions were more conserved than those of the LSC and SSC regions in the Bruguiera species, consistent with other mangrove chloroplast genomes (Li et al., 2019; Yang et al., 2019; Zhang, Zhong & Yuan, 2019; Shi et al., 2020). The IR regions had high GC content, concordant with other mangrove plants (Li et al., 2019; Yang et al., 2019; Zhang, Zhong & Yuan, 2019; Shi et al., 2020). In addition, a contraction of the IR-SSC boundary region was found in B. parviflora (163,228 bp); as a result, its chloroplast genome size was smaller than those of B. cylindrica, B. hainesii and B. sexangula (164,170–164,297 bp). There was an overlap between ycf1Ψ and ndhF in the SSC/IRB boundary region of B. parviflora, similar in several plant chloroplast genomes (Munyao et al., 2020; Su et al., 2020). These are common evolutionary events that result in size variation of chloroplast genomes in land plants (Wicke et al., 2011). Moreover, the genes adjacent to LSC/IR and SSC/IR boundaries are rps19, rps19 pseudogene, ycf1 and ycf1 pseudogene with variation in pseudogene length, similar in many land plants (Gichira et al., 2019). In contrast, the boundary of LSC/IRA junction of K. obovata, which is a true mangrove species in the family Rhizophoraceae, was near the rpl22 gene resulting in a shorter LSC and larger IR to give an overall larger chloroplast genome (Yang et al., 2019), revealing the variation of the IR junction among mangrove species during evolution.
A large number of repeats and SSRs were identified in the chloroplast genomes, that were mostly distributed in the LSC region as this contains a large amount of intergenic sequence. No differences were found between B. cylindrica and B. hainesii for the four SSR primer pairs (Fig. S2). B. hainesii was reported to be a hybrid between B. gymnorhiza and B. cylindrica identified using nuclear DNA markers (CesA and UKN) (Ono et al., 2016). The high similarity in chloroplast sequence and lack of genetic variation between B. cylindrica and B. hainesii are consistent with the chloroplast of B. hainesii originating from B. cylindrica. Comparison of SSRs, based on the same criteria of the SSR identification, in B. sexangula grown in two different locations (Thailand and China) revealed intraspecific sequence variation. For example, the Thai sample contained 186 SSRs, while only 175 SSRs were previously reported in the Chinese sample using the same criteria for SSR identification (Shi et al., 2020). The overall pattern of chloroplast SSR loci in the Bruguiera species were mostly unique compared with Kandelia and Rhizophora species. Thus, these SSRs would provide a useful genetic resource for future molecular marker analyses among Bruguiera species.
The ML phylogenetic tree was constructed based on 59 conserved chloroplast coding-genes in 40 species. The genus Bruguiera and other mangrove species, K. obovata, R. stylosa and R. x lamarckii, constituted a monophyletic branch of the clade Rhizophoraceae. Our phylogenetic result is consistent with morphological characters and previous phylogenetic results based on nuclear DNA markers and some chloroplast genes (e.g., matK and rbcL) (Schwarzbach & Ricklefs, 2000; Lakshmi, Parani & Parida, 2002; Sheue, Yong & Yang, 2005; Sahoo et al., 2007; Duke & Ge, 2011). For example, a phylogenetic tree was previously constructed using 34 land plant species including Bruguiera species (B. cylindrica, B. exaristata, B. gymnorhiza, B. parviflora, and B. sexangula) based on the rbcL gene and trnL-trnF spacer (Schwarzbach & Ricklefs, 2000). The phylogenetic result of these two sequences presented B. gymnorhiza and B. sexangula in the same clade. Another phylogenetic tree was reported based on chromosome and RAPD markers in four Bruguiera species that clustered into two groups with B. gymnorhiza and B. sexangula in one group, and B. cylindrica with B. parviflora in the other group (Sahoo et al., 2007). These results confirm the close relationships of B. gymnorhiza and B. sexangula.
The Ka/Ks values for all shared protein-coding genes of the Bruguiera species were calculated and compared with the four assumed ancestors to assess gene selection pressure. Among the protein-coding genes, nonsynonymous substitutions (Ka) were more frequent than synonymous substitutions (Ks) in rps7 (11 non-synonymous sites of 13 variation sites) and rpl36 (six non-synonymous sites of eight variation sites). The Ka/Ks values of the rps7 and rpl36 genes were above 1.0 in all species indicating that these genes were under positive selection. The result of the rps7 gene is consistent with previous findings for many mangrove species and some land plants such as K. obovata, R. stylosa, R. mangle and Ananas comosus (pineapple) (Redwan, Saidin & Kumar, 2015; Han et al., 2020). The rps7 gene encoding small ribosomal protein seven is involved in the regulation of chloroplast translation (Fargo, Boynton & Gillham, 2001). The rpl36 gene was reported to be under positive selection in many mangrove species such as Pemphis acidula, and Sonneratia ovata (Shi et al., 2020). Knockout of the rpl36 gene in tobacco results in severe morphological aberrations, poor photoautotrophic growth and low translational efficiency (Fleischmann et al., 2011). Therefore, the rps7 and rpl36 genes might be candidates for improving stress tolerance in mangrove species in dynamic and rapidly changing coastal environments.
Conclusions
This study reports the whole chloroplast genome sequences of the five Bruguiera mangrove species. The comparative analyses among them provide insights into the chloroplast genome evolution in the genus Bruguiera. Evidence from the Bruguiera chloroplast genomes confirms that B. hainesii, a hybrid species between B. cylindrica and B. gymnorhiza, has inherited the chloroplast from B. cylindrica. Translocation of rpl32 from the chloroplast to the nuclear genome was demonstrated to occur in only B. gymnorhiza; but not in other Bruguiera species. The comparison of the Bruguiera species in the family Rhizophoraceae based on phylogenetic relationships showed that B. gymnorhiza was closely related with B. sexangula, B. hainesii was confirmed to have inherited its chloroplast from B. cylindrica, and B. parviflora is a sister species of the other four Bruguiera species. Molecular markers were developed from SSRs and demonstrated to be useful in differentiating genotypes at both interspecific and intraspecific levels. The rps7 and rpl36 genes were found to be under positive selection in the Bruguiera species, and these genes might be important for stress tolerance. Therefore, these chloroplast genomes will provide useful genetic information for further analysis in Bruguiera and other mangrove species.
Supplemental Information
Sequences of a primer pair for testing rpl32 loss in five Bruguiera species.
List of chloroplast accession numbers and genes for phylogenetic analysis.
List of chloroplast accession numbers and genes for gene selective pressure analysis.
The read coverage of the rpl32 gene in five Bruguiera species.
The chloroplast genes with exons and introns in five Brugueira species.
SSRs of five Bruguiera species and three related mangrove species.
Shared compound and interrupted SSR loci with ≤100 bp among five Bruguiera species.
Ka/Ks values between Bruguiera species and assumed ancestors.
Analysis of PCR products by 1% agarose gel electrophoresis. A gel purified PCR products of a primer pair, ndhF-F and rpl32-R (Table S2) in five Bruguiera chloroplasts.
Lane M1 and M2: DNA size marker, Lane 1: PCR product in Bruguiera cylindrica (1,202 bp), Lane 2: PCR product in B. gymnorhiza (no product), Lane 3: PCR product in B. hainesii (1,202 bp), Lane 4: PCR product in B. parviflora (1,199 bp) and Lane 5: PCR product in B. sexangula (1,169 bp).
Analysis of SSR markers. A set of 20 of SSR markers were used to estimate the genetic variation among five Bruguiera species.
Y-axis is nucleotide size. A1–A5: SSR product of cpSSR1-F and cpSSR1-R primers (Table S3) in Bruguiera cylindrica, B. gymnorhiza, B. hainesii, B. parviflora and B. sexangula, respectively. A6–A10: SSR product of cpSSR2-F and cpSSR2-R primers in B. cylindrica, B. gymnorhiza, B. hainesii, B. parviflora and B. sexangula, respectively. A11–A15: SSR product of cpSSR3-F and cpSSR3-R primers in B. cylindrica, B. gymnorhiza, B. hainesii, B. parviflora and B. sexangula, respectively. A16–A20: SSR product of cpSSR4-F and cpSSR4-R primers in B. cylindrica, B. gymnorhiza, B. hainesii, B. parviflora and B. sexangula, respectively.