Characterization of phenylalanine ammonia-lyase genes facilitating flavonoid biosynthesis from two species of medicinal plant Anoectochilus
- Published
- Accepted
- Received
- Academic Editor
- Rogerio Sotelo-Mundo
- Subject Areas
- Biotechnology, Molecular Biology, Plant Science
- Keywords
- Phenylalanine ammonia-lyase, Anoectochilus formosanus, Anoectochilus roxburghii, Subcellular localization, Stress
- Copyright
- © 2022 Yang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Characterization of phenylalanine ammonia-lyase genes facilitating flavonoid biosynthesis from two species of medicinal plant Anoectochilus. PeerJ 10:e13614 https://doi.org/10.7717/peerj.13614
Abstract
Background
Anoectochilus roxburghii and Anoectochilus formosanus, belong to the Anoectochilus genus, have been used for Chinese herbal drugs as well as health food. Phenylalanine ammonia-lyase (PAL), the key enzyme in primary metabolism and phenylpropanoid metabolism, produces secondary metabolites (flavonoids) in plants, which are beneficial for the biosynthesis of phenylpropanoid metabolites.
Methods
The PAL genes were cloned from A. formosanus and A. roxburghii according to our previous transcriptomic analysis. The PALs were introduced into pCAMBIA2300-35S-PAL-eGFP to generate 35S-PAL-eGFP. The constructs were further used for subcellular localization and transgenic Arabidopsis. The expression of AfPAL and ArPAL under precursor substance (L-Phe), NaCl, UV, and red-light were analyzed by real-time quantitative PCR (RT-qPCR).
Results
AfPAL and ArPAL , encoding 2,148 base pairs, were cloned from A. formosanus and A. roxburghii. The subcellular localization showed that the ArPAL and AfPAL were both localized in the nucleus with GPF. Quantitative RT-PCR analysis indicated that the ArPAL and AfPAL genes function in the phenylalanine pathway as well as response to induced conditions. Overexpression of the AfPAL and ArPAL could increase flavonoids and anthocyanin content in the transgenic Arabidopsis.
Discussion
The results suggest that AfPAL and ArPAL play a crucial role in the flavonoid biosynthesis in Anoectochilus. Also, our study provides new insights into the enrichment of secondary metabolites of traditional Chinese medicines A. formosanus and A. roxburghii, which can improve their medicinal active ingredients and be used for drug discovery in plants.
Introduction
Anoectochilus is a specie of orchidaceae family and possesses various pharmaceutical constituents, which plays an important role in cancer treatment (Lang et al., 1999; Shyur et al., 2004; Yang et al., 2014; Yu et al., 2017). Currently, Anoectochilus formosanus and Anoectochilus roxburghii which are obviously different in morphology, are both widely used in cultivation or tissue culture for rapid propagation (Fig. 1, Du et al., 2000; Shiau et al., 2002; Zhang et al., 2015). However, the synthesis and catabolism of the pharmaceutical constituents including flavonoid, polysaccharides, glycoside derivative kinsenoside, and steroids in the cultivated Anoectochilus is primary and produced by secondary metabolites (Dai et al., 2009), which is different from wild plants (Du et al., 2000). It’s significant to promote the accumulation of flavonoids of two medicinal plants Anoectochilus in artificial cultivation or tissue culture.
Figure 1: The morphological characteristics of A. formosanus and A. roxburghii.
(A) and (B) represent A. formosanus and A. roxburghii; 1, 2 and 3 represents complete stool, leaf and stem; The leaf and stems of A. formosanus were white and pink, while those of A. roxburghii were golden yellow and green.Phenylalanine ammonia-lyase (PAL, EC 4.3.1.24) is essential for connecting of primary and phenylpropanoid metabolism in plants. The PAL controls the speed of the first step in the biosynthesis of phenylpropanoid metabolites, the nonoxidative deamination of phenylalanine to trans-cinnamic acid and ammonia (Lois et al., 1989; Nugroho, Verberne & Verpoorte, 2002). Subsequently, phenylpropanoids will produce several secondary metabolites, such as flavonoid, phytohormone, anthocyanin, lignin, phytoalexin, and benzoic acid (Fig. S1, Jorrin & Dixon, 1990; Jin et al., 2013). To date, the PAL genes have been cloned and characterized by homologous amplification and rapid amplification of cDNA ends (RACE) from variant medicinal plants, such as Dendrobium (Jin et al., 2013), Artemisia annua (Zhang et al., 2016a; Zhang et al., 2016b), Fagopyrum tataricum (Li et al., 2012) and Ginkgo biloba (Cheng et al., 2005). However, genome information is not available for homologous amplification for any of the Anoectochilus species. The expression of the PAL gene and activity of the PAL protein are found to be responsive to light quality, salinity, drought, wounding, and related to secondary metabolites accumulation in other plants (Nakazawa et al., 2001; Zhang et al., 2012; Zhang et al., 2016a; Zhang et al., 2016b).
Given the importance of A. formosanus, A. roxburghii, and the active compounds in these Anoectochilus, it is essential for functional studies into the medicinal plant. In the present study, the PAL genes were cloned from A. formosanus and A. roxburghii according to our transcriptional analysis. After bioinformatics analysis, the expression of the AfPAL and ArPAL genes in response to precursor substance (L-Phe), NaCl, UV, and red-light were detected by real-time quantitative PCR (RT-qPCR), respectively. The subcellular localization and heterologous expression of the AfPAL and ArPAL genes were performed. These results demonstrate that the AfPAL and ArPAL genes play an important role in flavonoids biosynthesis in Anoectochilus.
Materials & Methods
Sample preparation
The seedlings of A. formosanus and A. roxburghii were surface sterilized using 10% NaClO for 5 mins and plated onto MS medium in a chamber under a 12 h light/12 h dark at 28 °C and 60–80% humidity condition. The 4-month-old seedlings were transferred into a plastic mesh grid for aquaculture with Hoagland’s nutrient solution. The seedlings were transferred into a plastic mesh grid for aquaculture. Phe and NaCl were added into the nutrient solution with a final concentration of 4 mg/L and 100 mmol/L. The seedlings were also transplanted into plastic pots cultivated with nutritional soil and vermiculite (3:1), and then induced under a 253.7 nm UV and 650 nm red light. The leaves samples were collected from each replicate at 0 (control), 0.5, 1, 2, 4, 8, 12 h of the Phe, NaCl, UV, and red-light induction, respectively. The RNA was extracted with Qiagen RNeasy plant mini kit (Qiagen, China), following cDNA were revere transcribed by PrimeScript RT reagent Kit (Takara China).
Cloning of the PAL gene
The open reading frame (ORF) of the AfPAL and ArPAL gene from cDNA were amplified based on the annotation of RNA-seq by specific primers (5′-ATGGACCATGCTAGGGAG AACG-3′/5′-CTAGCAAATAGGGAGAGGAGCTTCA-3′) (http://www.premierbiosoft.com/primerdesign/). The amplified fragments were purified using Universal DNA Purification Kit (Tiangen, China), added dATP in the tail of sequences using the TaKaRa TaqTM (TakaRa, China), cloned into pMD19-T vector (TakaRa, China), and sequenced by Shanghai Sangon Biotech Co., Ltd (China).
Bioinformatic analysis
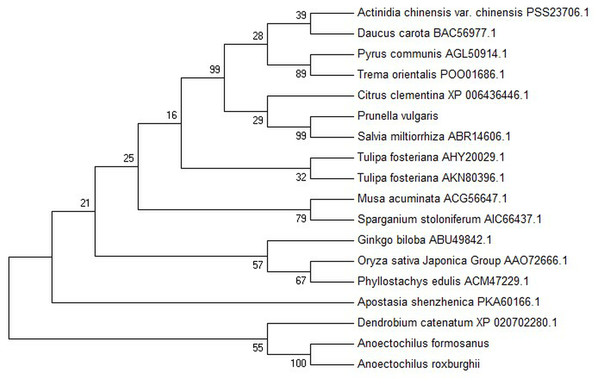
The sequencing of the AfPAL and ArPAL genes were aligned for gene structure using blast on NCBI website (http://www.ncbi.nlm.nih.gov) and used for the analysis of physical and chemical properties, secondary structure, functional domains, and genetic structure of the putative proteins by using ProtParam (http://web.expasy.org/protparam), GOR IV (http://npsa-pbil.ibcp.fr/cgi-bin/npsa_automat.pl?page=npsa_gor4.html), TMHMM Server v. 2.0 (http://www.cbs.dtu.dk/services/TMHMM-2.0/) and SWISS-MODEL (https://swissmodel.expasy.org/) software or databases, respectively. Phylogenetic analysis among the putative amino acid sequences of the AfPAL and ArPAL proteins at the NCBI database were analyzed using the method of maximum likelihood of 1,000 bootstrap replicates by using MEGA7.0 software (https://www.megasoftware.net/). The evolutionary distances were computed by using the Poisson correction method.
Vector construction
A pair of homologous arms (the lowercase bases) primers (5′-catttggagaggacagggtacccggg ATGGACCATGCTAGGGAGAACG-3′/5′-tcgcccttgctcaccatggtactagtGCAAA TAGGGAGAGGAGCTTCA-3′) was designed to amplify the ORFs of the AfPAL and ArPAL genes without termination codon for homologous recombination. The amplified PCR were inserted into the expression vector pCAMBIA2300 using CloneExpress One Step Cloning Kit (Vazyme, China) respectively, to generate a set of expression vectors bearing fusion genes between the AfPAL and ArPAL genes and the enhanced green fluorescent protein gene eGFP, AfPAL/ArPAL-eGFP (Fig. S2).
RT-qPCR
A pair of specific primers (5′- AGCAAGATTACGCCTTGCCT-3′/ 5′-AAGGCCTCTACTGCGTTGAC-3′) was designed to amplify a 152 bp fragment of the AfPAL and ArPAL genes. Another pair of specific primers (5′-CGGGCATTCACGAGACCAC-3′/5′-AATAGACCCTCCAATCCAGACACT-3′) was designed to amplify a 221 bp fragment of the internal reference gene Actin2 (Zhang et al., 2012). The PCR reaction was conducted on SsoFast EvaGreen Supermix (Bio-Rad, Hercules, CA, USA) according to the protocol. The 2−ΔΔCT method was used to normalize the expression between the internal reference and the PAL genes (Livak & Schmittgen, 2001). The data was further analyzed by IBM-SPSS software (http://www-01.ibm.com/software/analytics/spss/).
Subcellular localization
The 35S-PAL-eGFP recombined vectors were attached onto gold particles (φ = 60 µm) by the spermidine and CaCl2 method with 35S-eGFP empty vector as control (Yu et al., 2018). Onion bulbs were surface sterilized with 75% (v/v) ethanol. The healthy fourth to sixth scales were cut into 2 × 2 cm, cultured on Murashige and Skoog’s (MS) medium for 4–6 h under dark at 28 °C, and then bombarded using helium biolistic gun (Bio-Rad, USA), incubated for 24 h at 28 °C under dark condition (Sun et al., 2021). The fluorescence signal was detected by a confocal microscope (Nikon, Japan; Yang et al., 2019).
Transformation of Arabidopsis
The pCAMBIA2300-35S-PAL-eGFP plasmid was mobilized into Agrobacterium tumefaciens strain EHA105 and used to transform wild-type Arabidopsis as described (Sun et al., 2020). The transformed lines were screened on 1/2 MS medium supplemented with 35 mg/L kanamycin (Sigma, St. Louis, MO, USA). The homozygous lines were identified by PCR amplification with specific primers (5′- CATTTGGAGAGGACAGGGTACC-3′/5′-CTAGCAAATAGGGAGAGGAGCTTCA-3′) for AfPAL and ArPAL.
Flavonoid quantification
The leaf of T3 lines and wild type were dried and ground to be extracted by 95% alcohol in an ultrasonic instrument at 25 °C for 0.5 h. The extracts were filtered, and the residues were dissolved in 95% alcohol. The residues were filtered again. The filtrates were combined, and solvents were removed under reduced pressure using the rotavapor R-210 (BUCHI, Switzerland) to yield the extract. The template samples were detected using the reagent color-developing method (NaNO2-Al(NO3)3–NaOH). The above stock solutions 1 ml were added with 0.4 mL 5% NaNO2 for 5 min. The 10% Al(NO3)3 0.4 ml were added to the reaction. After standing for 5 min, 4% NaOH 4 ml were used to color. The reactions were incubated for 20 min. The quantitative values were determined with a UV-1800 spectrophotometer at 420 nm. The content of total flavonoids was calculated as: where, A420 was the absorbance at 420 nm, V repreents total volume of the extract, mM was the extraction quality from the leaf of each sample (1 g), d repreents the dilution multiple (Chen et al., 2007).
Anthocyanin measurement
The leaves of T3 lines and wild type were pulverized to fine powder in liquid nitrogen, extracted with acidified (1% HCl) methanol, and incubated dark with shaking for 48 h. Later, it was centrifuged at 4,000 g for 10 min following the protocol described by Tanaka et al. (1997). The supernatant was used to measure absorbance at 535 nm in a UV-1800 spectrophotometer. The anthocyanidin content was indicated by absorption value.
Results
Cloning the AfPAL and ArPAL genes
Based on RNA-seq information, we designed the specific primers to amplify the AfPAL and ArPAL. The fragments of more than 2,148 bp were amplified from the cDNA library of A. formosanus and A. roxburghii, respectively (Fig. S3, MK387342 and MK387343). The constructs were verified by PCR and sequencing; the fragment from the cDNA of A. formosanus and A. roxburghii showed high homology to reported PAL bioinformatics-predicted sequences (Fig. S4).
Proteins sequence analysis
The amino acid sequences of the AfPAL and ArPAL proteins were highly homologous with that from Phalaenopsis equestris (XP_020579635.1) and Dendrobium huoshanense (Figs. 2 and 3). The AfPAL and ArPAL proteins both contained 715 amino acids with a molecular weight 77.4 kDa, isoelectric point pI 6.18 and 6.26, grand average of hydropathicity (GRAVY) −0.104 and −0.103. The predicted secondary structure of these two proteins contained 48.53% and 48.67% α-helices, 10.07% and 9.79% extended strands, 41.40% and 41.54% random coils, respectively. Their three-dimensional structural model contained all the α-helices, extended strands, and random coils (Fig. S5). Most of these properties of the AfPAL and ArPAL proteins were similar to the PePAL of Phalaenopsis equestris.
Figure 2: The structural functional domain of the PAL genes among A. formosanus, A. roxburghii and Phalaenopsis equestris.
Identical and conserved amino acid residues are denoted by black (100%), gray (66.6%) and white (0%) backgrounds, respectively. The boxes with solid line represent the phenylalanine and histidine ammonia lyase signature, the boxes with dotted line represent the possible phosphorylation sites, the asterisks represent the active sites, the solid dots represent strictly conserved residues, the circles represent the deamination sites and sharp corners represent the catalytic active sites.Figure 3: Phylogenetic tree among the putative proteins of A. roxburghii, A. formosanus and deposited functional PAL proteins of other plants.
Conserved domain and Phylogenetic relationship
The conserved domain of the phenylalanine and histidine ammonia lyase signature (GTITASGDLVPLSYIA) and the active site Ala-Ser-Gly tripeptide forming the MIO group (3,5-dihydro-5-methylene-4H-imidazole-4-one) were found in position 197-213 and 201-203, respectively. Meanwhile, the strictly conserved residues, Y109, L137, S202, N259, Q347, Y350, R353, F399, and Q487, were found in the AfPAL and ArPAL protein, respectively. Moreover, the deamination sites such as L-205, V-206, L-255, and A-256, catalytic active sites such as N-259, G-260, NDN (381–383 aa), H-395 and HNQDV (485–488 aa), and the possible phosphorylation site such as VAKRVLTF (542–549 aa) were found in both AfPAL and ArPAL (Fig. 2). Multiple alignment and phylogenetic analysis showed that the putative AfPAL and ArPAL proteins were clustered into the same sub-group with the deposited functional PAL proteins of Dendrobium huoshanense (Fig. 3), indicating that the PAL proteins from A. formosanus and A. roxburghii are members of the PAL family.
Relative expression level under induction
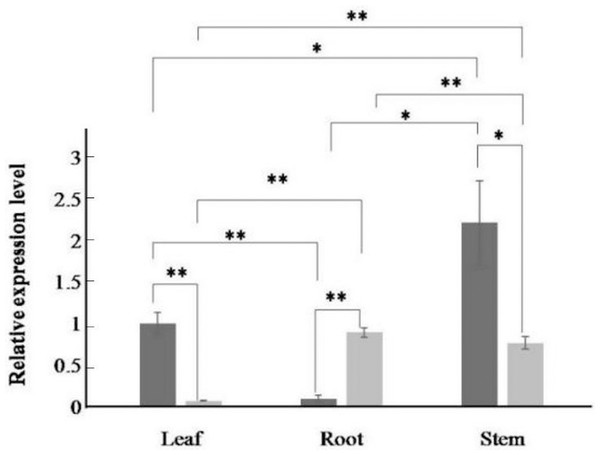
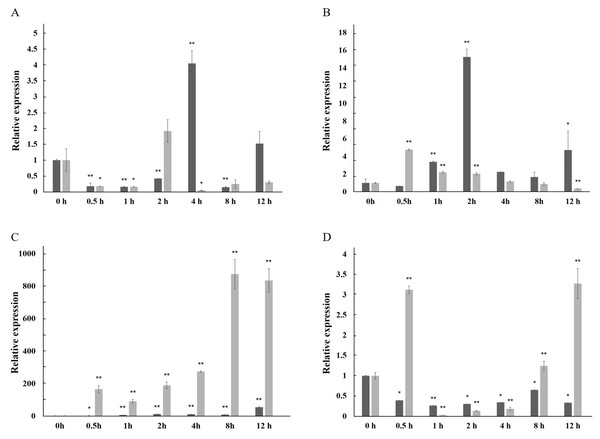
The expression of the AfPAL gene in the stem was highest, about twice than the leaf, and it was the 20 times than root from A. formosanus (P ≤ 0.05; Fig. 4). The expression of the ArPAL gene in the root was similar to that of the stem, about 10 times higher than the leaf from A. roxburghii (P ≤ 0.05; Fig. 4). And the expression of the PAL gene was similar in root from A. formosanus, leaf, and stem from A. roxburghii. The expression was downregulated significantly in A. formosanus and A. roxburghii, and there was a valley value at 1 h in response to Phe stress at the beginning. Then, the expression was upregulated in A. formosanus and A. roxburghii after 1 h, and reached their peaks at 4 h and 2 h, respectively. Then the expression plummeted, only one-tenth or even less with the control (Fig. 5A). Under the NaCl stress, the expression was upregulated significantly in the two species and reached their valleys at 2 h (15.24 times) and 0.5 h (4.77 times), respectively (Fig. 5B). In response to the UV stress, the overall trend of the expression of the PAL genes was upregulated, and there was a peak value at 12 h and 8 h, respectively (54.49 times and 873.89 times, Fig. 5C). The expression was downregulated significantly in A. formosanus and reached its valley at 1 h in response to red light stress. In contrast, it was changed but not regular in the A. roxburghii (Fig. 5D).
Figure 4: Relative expression levels of AfPAL and ArPAL genes among different organs.
The darker columns represent A. formosanus, the lighter columns represent A. roxburghii. The asterisk (*) and double asterisk (**) stand for significance with the control at 0.05 and 0.01 levels, respectively.Figure 5: Relative expression level of the PAL gene under the stress in A. formosanus and A. roxburghii.
(A) under the Phe stress; (B) under the NaCl stress; (C) under the UV; D: under the red-light stress. The asterisk (*) and double asterisk (**) stand for significance with the control at 0.05 and 0.01 levels, respectively.Subcellular localization
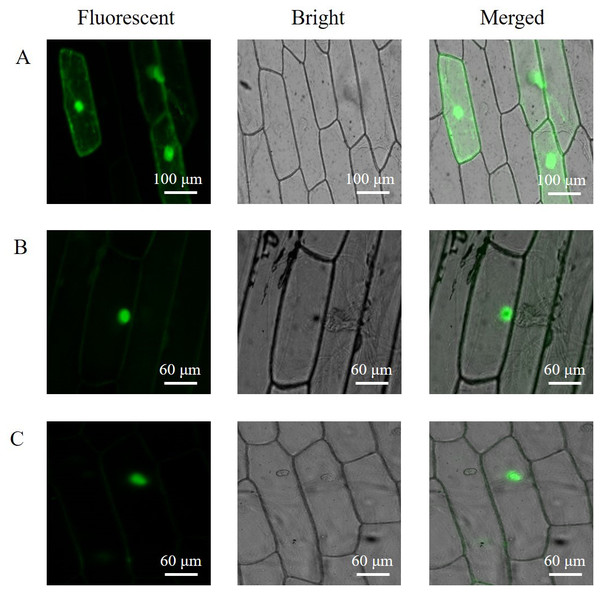
The subcellular localization of the PAL proteins was analyzed using the GFP as a reporter in transient expression assays, and bacterial cells carrying PAL-GFP plasmids were infiltrated into epidermal cells of onion. Confocal microscopy images demonstrated that the PAL-eGFP fusion protein was specifically distributed in the nucleus, whereas GFP alone showed ubiquitous distribution in the whole cell (Fig. 6).
Figure 6: Subcellular localization of PAL protein.
eGFP and PAL-eGFP fusion gene were driven under the control of the CaMV 35 Spromoter. (A) Epidermal cells of onion transformed by pC2300-35S-eGFP. (B) Epidermal cells of onion transformed by pC2300-35S-PAL-eGFP from A. formosanus. (C) Epidermal cells of onion transformed by pC2300-35S-PAL-eGFP from A. roxburghii.Overexpression of the PAL genes
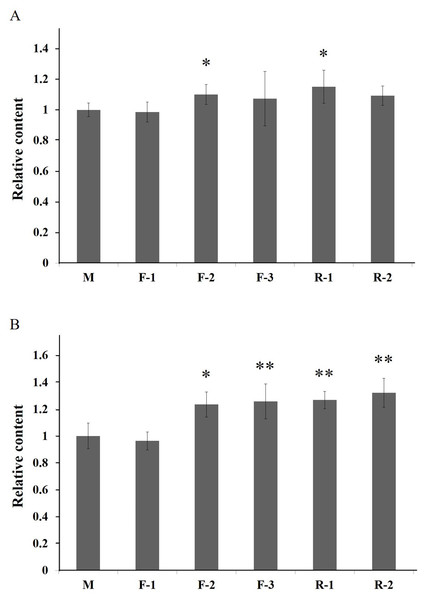
To investigate the function of PAL, the transgenic Arabidopsis of PALs were generated. In the T1 generation, five positive plants of three lines (F1-3) transformed by gene AfPAL and two lines (R1-2) by ArPAL were screened on the selection medium. In the T2 generation, these lines were a single copy insertion with a ratio of 3:1 between the transformed genes and wild-type Arabidopsis (Fig. S6). In the T3 generation, homozygous lines without segregation were identified on the selection medium. The specific PCR amplification confirmed the altered genes from F1-3 and R1-2(Fig. S7). The flavonoid contents were significantly higher in lines F-2 and R-1, and the anthocyanin content was considerably higher in lines F-2, F-3, R-1, and R-2 (Fig. 7). The results revealed that the PAL genes were successfully expressed in five independent transgenic events. The flavonoids and anthocyanin contents in transgenic lines were higher than in wild-type.
Figure 7: Relative content of total flavonoids and anthocyanin of T3 Arabidopsis lines of gene PAL from A. formosanus and A. roxburghii.
(A) Relative content of total flavonoids of T3 Arabidopsis lines of gene PAL from A. formosanus and A. roxburghii. (B) Relative content of anthocyanin of T3 Arabidopsis lines of gene PAL from A. formosanus and A. roxburghii. The asterisk (*) and double asterisk (**) stand for significance with the control at 0.05 and 0.01 levels, respectively.Discussion
The ORFs of the cloned AfPAL and ArPAL genes and the amino acid sequences of their putative proteins were highly homologous with the PAL gene and its putative protein in Dendrobium huoshanense as well as Phalaenopsis equestris (Figs. 2 and 3). The conserved domain of the phenylalanine, histidine ammonia-lyase signature, and the active site Ala-Ser-Gly tripeptide forming the MIO group was necessary for their function (Gao et al., 2008). All the active sites were highly conserved among the reported PAL proteins (Cheng et al., 2005; Gao et al., 2008; Jin et al., 2013; Li et al., 2012; Zhang et al., 2016a; Zhang et al., 2016b).
In many other plants, the expression of the PAL genes showed organic specificity, and the expression level was correlated to their accumulation of flavonoids (Fukasawa-Akada, Kung & Watson, 1996; Jin et al., 2013; Leyva et al., 1992; Zhang et al., 2016a; Zhang et al., 2016b). In this paper, RT-qPCR analysis revealed that the high expression of the PAL genes was found in the stem of A. formosanus and the root of A. roxburghii (Fig. 4). However, their differential expression was responsive to four stress or induction treatments. This result implies the different tolerance of these two species in the activities of PAL (Fig. 5). The expression of the PAL genes was intensely upregulated in response to NaCl and UV (Figs. 5B and 5C), which was consistent with observations of other plants under stress conditions (Bell et al., 2017; El, Wilson & Callahan, 2003; El-Shora, 2002; Fritzemeier & Kindl, 1981; Song & Wang, 2009). The range of the differential expression of the AfPAL gene was more extensive than the ArPAL gene under NaCl stress, but the content of the AfPAL gene was less than the ArPAL gene, conversely. In response to Phe and red-light induction, the expression of the PAL gene was downregulated, excepting a few sharp peaks (Figs. 5A and 5D). Similar results were found in other plants (Nakazawa et al., 2001; Bellini & Hillman, 1971; Edahiro et al., 2005; Heo et al., 2012). Therefore, it is preliminary concluded that the expression of the PAL gene is more sensitive to saline induction in A. formosanus than A. roxburghii, and the latter is probably more sensitive to UV induction.
Subcellular localization of PAL protein has been studied in different plants (Fukasawa-Akada, Kung & Watson, 1996; Herdt & Wiermann, 1978). The present study investigated the subcellular localization of PAL protein in a heterologous system (the chloroplast-free epidermal cells of onion) by confocal laser-scanning microscopical imaging of GFP fluorescence (Fig. 6). Transient expression of the PAL-eGFP fusion protein in the onion was targeted to the nucleus. The nuclei of five tree species with respect to the presence of flavanols (Feucht, Treutter & Polster, 2014). Flavonoids and at least two of the biosynthetic enzymes are located in the nucleus in several cell types in Arabidopsis (Saslowsky & Winkel-Shirley, 2005). The result might indicate a high association of PAL protein to the nucleus or nuclear membrane and raise the possibility of novel mechanisms of action for flavonoids.
In the overexpressing transgenic lines of Arabidopsis thaliana, the content of flavonoids and anthocyanin was significantly higher than those in wild-type control (Fig. 7). The increased contents of total flavonoids should be associated with the genetically modified anthocyanin metabolic pathway (Fig. S1). In addition, the content of total flavonoids and anthocyanins was higher in the Arabidopsis lines transformed by the ArPAL gene than those of the AfPAL. This result suggests that the activities of the proteins encoded by PAL genes might be differential between these two species. The transgenic tobacco with the overexpression PAL gene was developed in response to infection by tobacco mosaic virus and necrotrophic pathogens (Pallas et al., 2010; Way, Birch & Manners, 2011). Many reports indicated its critical function in the secondary metabolism of these plants (Lois et al., 1989; Nugroho, Verberne & Verpoorte, 2002).
Conclusions
The AfPAL and ArPAL genes’ expression showed organic specificity and the differential expression of the PAL genes in response to four treatments. The flavonoid metabolites of Arabidopsis transformed with AfPAL, and the ArPAL gene were increased, provided by the anthocyanin metabolic pathway. And the different effects of overexpressed Arabidopsis flavonoids were caused by different Anoectochilus of the PAL gene.
Supplemental Information
The fragments of the PAL gene from genomic DNA in A. formosanus and A. roxburghii
M represents marker; 1, 2 represents A. formosanus and A. roxburghi ai.
The sequeses of PAL unigene from A. formosanus and A. roxburghii
Identical and conserved bases are denoted by black (100%), gray (66.6%) and white (0%) backgrounds, respectively, and the box represent the ORF.
Predicted three-dimensional models of the putative proteins of the PAL genes between A. formosanus. A.roxburghii and P. equestris
Agrobacterium mediated PAL gene transfer in Arabidopsis form A. formosanus and A.roxburghii
A and B represent A. formosanus and A.roxburghii, respectively; black arrows represent living Arabidopsis (overexpression); white arrows represent dead Arabidopsis (wild type).
PCR detection of T3transgenic line
M: DNA molecular maker DL2000; +: Positive control (expression vecgtor pC2300-35S-PAL-eGFP); -: Negative control (untransformed line); F-1, F-2 and F-3: T3 line of gene PAL from A. formosanus; R-1 and R-2: T3 lines of gene PAL from A. roxburghii.
The AfPAL and ArPAL genes and protein sequences
AfPAL and ArPAL represent the sequence of phenylalanine ammonia-lyase gene from Anoectochilus formosanus and Anoectochilus roxburghii respectively; AfPAL and ArPAL represent the sequence of phenylalanine ammonia-lyase protein from Anoectochilus formosanus and Anoectochilus roxburghii respectively.
The raw data for Figures 5, 6, 8
The molecular and functional characterization Phenylalanine ammonia-lyase and their expression analysis in relation to flavonoid constituents from two species of medicinal plant Anoectochilus.