High temperature mortality of Wolbachia impacts the sex ratio of the parasitoid Ooencyrtus mirus (Hymenoptera: Encyrtidae)
- Published
- Accepted
- Received
- Academic Editor
- Joseph Gillespie
- Subject Areas
- Developmental Biology, Ecology, Entomology, Zoology, Environmental Impacts
- Keywords
- Reproductive modification, Symbiosis, Bagrada hilaris, Biological control
- Copyright
- © 2022 Power et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. High temperature mortality of Wolbachia impacts the sex ratio of the parasitoid Ooencyrtus mirus (Hymenoptera: Encyrtidae) PeerJ 10:e13912 https://doi.org/10.7717/peerj.13912
Abstract
Background
Wolbachia bacteria are estimated to occur in more than half of all insect species. In Hymenoptera, Wolbachia often manipulates its host’s reproduction to its own advantage. Wolbachia is likely the reason that males are rare in the uniparental Ooencyrtus mirus Triapitsyn & Power (Hymenoptera: Encyrtidae). The likelihood of producing male offspring can be increased by giving mothers a continuous supply of Bagrada hilaris (Burmeister) (Heteroptera: Pentatomidae) host eggs to parasitize for 2–3 weeks, by feeding the parents antibiotics, or by rearing parent wasps at high temperatures; all variables that have been shown to correlate with depleting Wolbachia titers in other organisms. The purpose of the current study was to determine whether thelytoky in O. mirus is due to Wolbachia, and if so, at what time in development the sex change occurs. We also wished to determine if Wolbachia removal results in the production of intersexes, as in some other hymenopterans. Finally, mating behavior was observed to see if and where it breaks down as a result of the species becoming thelytokous.
Methods
Females were collected from parental lines of O. mirus reared at 26, 30, 31, 32, 33, 34, and 36 °C. The offspring of these females were reared at 26 °C, and their sex-ratio was determined. In a subsequent experiment, the parental generation was switched between 26 °C and 36 °C during development to narrow down the critical period at which changes occurred that subsequently affected the sex-ratio of their offspring.
Results
The sex ratio was male biased in the offspring of O. mirus parents reared at 34 °C and 36 °C (high temperatures), even if the offspring themselves were reared at 26 °C. The constant temperature at which the percentage of males started to increase after two generations was 31 °C (10% males), rising to 39% males at 33 °C, and 100% males at 34 °C and 36 °C. Lasting more than 2 days, the critical period for the change toward a male biased sex ratio was during the second half of the parent’s development. Molecular diagnostic assays confirmed that O. mirus females contain Wolbachia and males do not. Examination of preserved males and male-female pairs under a dissecting microscope showed no signs of intersex characters. Observation of the mating behavior of live O. mirus showed that males initiate courtship by drumming their antennae on a female’s antennae, but after a few seconds, the females typically turn and walk away. However, a few instances of possible copulation were noted.
Conclusions
As hypothesized, the results indicated that thelytoky in O. mirus is likely mediated by Wolbachia bacteria. To maximize the population growth rate without generating males, the best temperature for mass rearing this species is 30 °C.
Introduction
Bagrada hilaris (Burmeister) (Heteroptera: Pentatomidae) is an invasive pest on brassica crops. Native to small parts of Africa, Asia, and the Middle East (Howard, 1906; Husain, 1925; Taylor, Bundy & McPherson, 2014), it invaded southern Europe in 1978 (Colazza, Guarino & Peri, 2004), and Los Angeles County, California, in the United States in 2008 (Arakelian, 2008). Since then, it has spread to other counties of California, and other southwestern states (Palumbo & Natwick, 2010; Bundy, Grasswitz & Sutherland, 2012; Vitanza, 2012; Perring et al., 2013; Reed et al., 2013). It also has established in Hawaii (Matsunaga, 2014), Mexico (Sánchez-Peña, 2014; Torres-Acosta & Sánchez-Peña, 2016; Hernández-Chávez et al., 2018), and Chile (Faúndez et al., 2016; Faúndez, Lüer & Cuevas, 2017) and recently in Argentina (Carpintero et al., 2021). According to Carvajal et al. (2019), its distribution has the potential of including all regions with a Mediterranean climate.
As part of an effort to identify potential biological control agents to aid in B. hilaris management, three hymenopteran egg parasitoid species were collected from B. hilaris eggs in the field in Pakistan (Mahmood et al., 2015). One of these species has recently been described as Ooencyrtus mirus Triapitsyn & Power (Triapitsyn et al., 2020). The biology of this new species has been under investigation to determine its potential for field release (Ganjisaffar, Power & Perring, 2021; Power, Ganjisaffar & Perring, 2020a; Power, Ganjisaffar & Perring, 2020b; Power et al., 2021).
Ooencyrtus mirus is a thelytokous species in which females are produced from unfertilized eggs. The current study investigates whether thelytoky in O. mirus is due to infection with a bacterium, Wolbachia. Transmitted from a female host to her offspring, Wolbachia are gram-negative α-proteobacteria (Rickettsiales: Anaplasmataceae) that are estimated to occur in more than half of all insect species (De Oliveira et al., 2015). In Hymenoptera, Wolbachia has been shown to manipulate its host’s reproduction in a way that favors survival of the bacterium (Stouthamer, Breeuwer & Hurst, 1999). One of the ways Wolbachia achieves this is by inducing parthenogenesis in which haploid eggs that would normally develop as males become diploid females. This has been documented in multiple species and results from Wolbachia modifying the first mitotic division in the egg, so that the resulting nucleus is diploid (Stouthamer & Kazmer, 1994; Doremus & Hunter, 2020).
If parthenogenesis in O. mirus is due to Wolbachia, then removal of the Wolbachia should result in a higher proportion of male offspring. Wolbachia can be removed in at least three ways. The first is by feeding antibiotic-laced honey to the adult female hosts (Stouthamer, Luck & Hamilton, 1990). The second is by maintaining the host colony at a temperature that is high enough to kill the Wolbachia but not the insects (Stevens, 1989; Stouthamer, Luck & Hamilton, 1990; Van Opijnen & Breeuwer, 1999). A third way is to provide the parasitoids a continuous supply of host eggs, which depletes a mother’s Wolbachia supply as she lays her eggs faster than microbe titers can recover (Lindsey & Stouthamer, 2017). In this study, we checked for the effect of Wolbachia removal on offspring sex ratio by rearing O. mirus at high temperatures. We narrowed down the temperature at which the change in sex occurs, and further narrowed down the critical developmental time period in which it occurs.
Besides increasing the proportion of males, in some species, the removal of Wolbachia causes intersexes to arise in some of the host offspring; e.g., the sawfly Diprion pini (L.) (Hymenoptera: Diprionidae) (Pistone et al., 2014), the moth Ostrinia scapulalis (Walker) (Lepidoptera: Crambidae) (Sakamoto et al., 2008), and egg parasitoids of the genus Trichogramma (Hymeoptera: Trichogrammatidae) (Tulgetske & Stouthamer, 2012). Intersex individuals consist of a single sexual genotype but have both male and female sex characteristics (Tulgetske & Stouthamer, 2012).
As there are advantages of thelytokous reproduction for the application of wasps in biological control (Stouthamer & Kazmer, 1994), in some cases Wolbachia-infected females produce substantially fewer offspring than their arrhenotokous conspecifics. In many species of the genus Trichogramma, Wolbachia-infected thelytokous cultures can be rendered arrhenotokous by “curing” the wasps of their Wolbachia infection. Such cured lines often produce more female offspring than the Wolbachia-infected lines (Stouthamer & Luck, 1993). Here we want to determine the effects of Wolbachia infection and it’s removal on the offspring production of the parasitoid wasp O. mirus in order to determine: (1) if Wolbachia is the cause of thelytokous reproduction in this species; (2) if removal of the infection allows the establishment of an arrhenotokous population of this species; and (3) how rearing temperatures affect offspring sex ratios and the production of intersexes, in order to determine the best rearing temperature for this species’ application in biological control.
Material and Methods
Insect rearing
Ooencyrtus mirus and B. hilaris were reared according to Power, Ganjisaffar & Perring (2020a). The O. mirus colony was established in 2016 in the quarantine facility at the University of California, Riverside, from individuals sent from the Toba Tek Singh District in the Punjab Plain of Pakistan (Mahmood et al., 2015). The parasitoids were reared in upside-down ClickClack® containers with rubber-stopper-filled holes for adding host egg cards and applying streaks of honey. These containers were maintained at room temperature of 23 °C with natural light.
The B. hilaris colony was started from individuals collected in Riverside, California in 2010, and refreshed annually with field-collected insects to maintain genetic diversity. The B. hilaris colonies were reared in tent-style cages (BugDorm®-2120, MegaView Science Co., Taiwan) in greenhouses set at 24−31 °C, and fed with broccoli (Brassica oleracea L. variety Italica), green mustard (Brassica juncea (L.)), and mizuna (Brassica rapa L. variety Japonica) seedlings grown in 10 × 10 cm plastic pots. As needed for egg collection, adults were brought to an insectary room where they were kept in round plastic containers (Durphy® Packaging Co., Warminster, PA, USA) at 30 ± 1 °C, 40–50% RH and 14:10 L:D and fed organic broccoli florets. From these containers, eggs were collected daily, glued (Elmer’s® Products, Inc., Columbus, OH, USA) to small pieces of card stock, and transferred to the O. mirus colony.
Effect of temperature on sex ratio
To evaluate the impact of temperature on parasitoid sex ratio, B. hilaris host eggs were collected from the Durphy® containers and glued to card stock. These eggs were provided to parasitoid females from the colony (F0) in nine cm height ×2 cm diameter glass vials. The vials were streaked with honey to provide food for the parasitoids. Depending on the number of eggs and the number of parasitoids available, parasitism was allowed to proceed for various amounts of time to obtain maximum parasitism, but not support superparasitism.
Typical of Encyrtidae, each O. mirus egg has a pedicel that protrudes through the host chorion and serves as a respiratory tube for the developing larva (Maple, 1947). A stereomicroscope was used to identify host eggs that had only one pedicel, indicating a single parasitoid egg. Each of these parasitized eggs (F1) was cut out on its section of card stock, placed inside a size 0 gel capsule (Capsuline®, Dania Beach, FL, USA), and subsequently reared at 26, 30, 31, 32, 33, 34, or 36 °C. The highest temperature (36 °C) was chosen because previous studies showed it to be the temperature at which development is quickest in O. mirus (Power, Ganjisaffar & Perring, 2020a). All treatments yielded 100% F1 females and these were given access to B. hilaris eggs to produce an F2 cohort, all of which were reared at 26 °C. All single-pedicel parasitized eggs were checked daily until the parasitoid emerged, at which time the sex and the total number of individuals was recorded. Data for the temperatures (31−33 °C) that yielded intermediate percentages of males (greater than 0% and less than 100%) were compared using Pearson χ2 tests in R (R Core Team, 2021).
Critical time period for sex determination
Two separate tests were conducted to narrow down the critical period when exposure of a developing O. mirus female to high temperature (36° C) dramatically increased the likelihood that her progeny would be male rather than female. In the first test, 3-day-old naïve O. mirus females (F0 generation) were exposed to 1-day-old B. hilaris eggs glued to card stock at a ratio of 13 wasps to 25 host eggs in a glass vial for 2.25 h. The egg card was removed, and eggs with a single pedicel (F1 generation) were selected under a stereomicroscope and divided evenly into 7 groups. These procedures were repeated for two more days until each of the 7 groups had eleven single-pedicel eggs. Parasitized eggs from different days were kept separate. For each collection day, the vials were labeled 1 through 7, along with the date. The vial labeled as #1 was placed in a 36 °C growth chamber and the other six vials in a 26 °C growth chamber. Two days later, vial #1 was transferred to 26 °C and vial #2 was transferred to 36 °C. The same procedure was followed every subsequent 2 days, through day 12; the vial at 36 °C was returned to 26 °C, and the next sequentially numbered vial was transferred to 36 °C. All F2 were reared at 26.
As the total immature developmental time is 14-15 days at 26 °C (Power, Ganjisaffar & Perring, 2020a), this gave each group a chance to be at 36 °C for a different 2-day period, or 1/7 of the developmental time. When the adult wasps emerged, the number and sex were recorded. After 3 days, they were provided 1-day-old B. hilaris eggs at the rate of 5 host eggs per O. mirus female for 24 h. Again, the eggs with one pedicel (F2 generation) were separated out, and reared in glass vials at 26 °C until the offspring emerged. The number and sex of these F2 adults were recorded.
Since the F2 generation in the first test was 100% female (see Results), we suspected that the length of time at the high temperature was not sufficient to kill the Wolbachia, regardless of when the parasitoids were exposed to 36 °C. Therefore, a second test was conducted to expose the individuals for longer time periods. In this study, the F1 eggs were divided into four groups: (A) constant 36 °C; (B) 36 °C for four days (half of the total developmental time at 36 °C), and then transferred to 26 °C; (C) 26 °C for seven days (half the developmental time at 26 °C), and then transferred to 36 °C; and (D) constant 26 °C. Procedures for the F2 generation were the same as in the first critical period test, with the F2 generation reared at 26 °C.
Wolbachia detection
In order to rule out infection with two common reproductive endosymbionts, Cardinium and Rickettsia we used established molecular assays described by Weeks, Velten & Stouthamer (2003) and Gottlieb et al. (2006), respectively. Each assay yielded a PCR product for its positive control (Aspidiotus nerii (Bouché) and Bemisia tabaci (Gennadius), respectively), but none for any of six O. mirus females tested. We screened for the presence of Wolbachia in O. mirus females and males using a diagnostic polymerase chain reaction (PCR) based on the bacterial 16S rRNA gene (Werren & Windsor, 2000). Females and males for these analyses were offspring of adults reared at 26 °C. Additional males were obtained by rearing parents at 36 °C, by constant exposure of O. mirus females to B. hilaris eggs for more than two weeks, and by 24-hour exposure of O. mirus females to Euschistus conspersus Uhler (Hemiptera: Pentatomidae) eggs, an alternate host of O. mirus (Power, Ganjisaffar & Perring, 2020b). Ooencyrtus mirus adults were euthanized in 95% ethanol in microcentrifuge tubes. DNA was extracted using an established Chelex® resin-based method. The wasps were moved from the ethanol onto sterile filter paper to let the ethanol evaporate, after which they were placed, three per tube, into clean tubes containing 2 µl proteinase K (>600 mAU/ml; QIAGEN #19131), and ground using a sterile glass pestle. Following maceration, 60 µl of a 5% suspension of Chelex® 100 (Bio Rad, Hercules, CA, USA) in water was added. The reactions were incubated in a water bath at 55 °C for 1 h, and then in a second water bath at 99 °C for 10 min. Each sample then was spun at 14,000 rpm for 4 min to pellet the Chelex® resin, and 50 µl of the DNA-containing supernatant was transferred to a new tube. Extracted DNA was stored at −20 °C until used in the PCR.
Diagnostic PCRs were conducted in 25 µl volumes containing 1x Thermopol® Buffer (New England Biolabs®, Ipswich, MA, USA), 1.5 ml BSA (10 mg/ml; New England Biolabs®), an additional 1 mM MgCl2, 0.2 mM each dNTP, 2 µl of template DNA, and 0.4 mM each of the primers W-Specf/W-Specr (Werren & Windsor, 2000). Thermocycling followed (Werren & Windsor, 2000) with the exception that we ran 42 cycles, and the extension temperature was lowered to 68 °C. The presence/absence of PCR products was checked using standard gel electrophoresis.
Intersexuality
Forty-one males and 28 male–female pairs of O. mirus, all sourced from adult O. mirus females exposed to a continuous supply of B. hilaris eggs for two weeks or more, were euthanized in 95% ethanol in microcentrifuge tubes. Within each pair, the male and female were siblings that emerged on the same day in the same vial. Each preserved individual was checked under a Leica Wild M10 Stereo/Dissecting microscope for evidence of intersexuality. The features checked included setal length on both antennae, abdomen coloration, and genitalia. Male antennal setae are longer than the antennal width, whereas female setae are shorter than the antennal width. The proximal half or more of the female gaster is bright yellow, whereas the male abdomen has subtle bands of black and pale yellow (Triapitsyn et al., 2020). The female ovipositor rests in a longitudinal groove in the abdomen, whereas the male genitalia does not. The triangular tip of the ovipositor extends only slightly beyond the tip of the abdomen, whereas the male genitalia extends well past the tip of the abdomen (Triapitsyn et al., 2020).
Mating behavior
Males and females were separated within 24 h after emergence. Three O. mirus males were added to a vial with three O. mirus females and observed under a stereoscope with fiber optics (cool) lights for 10 min. This was repeated for a total of 12 observation times with fresh males and females, each time in a clean vial. The females were 3 days old because this is the day females reach full egg laying capacity (Power et al., 2021). The males were different ages, ranging from 0 to 4 days old and at least two sets of three males of each age were observed.
Results
Effect of temperature on sex ratio
Female parents reared at constant 26 °C and 30 °C produced no male offspring (Table 1). A low percentage of males (2–10%) emerged from parents reared at 31 °C and 32 °C, which were not significantly different from each other (P = 0.098, χ2 = 2.74, df = 1). The percentage of males at 33 °C (39%) was significantly higher than those at 31 °C (P <0.001, χ2 = 20.17, df = 1) and 32 °C (P = 0.009, χ2 = 6.78, df = 1). Parents reared at 34 °C and 36 °C produced 100% male offspring. Overall, the percentage of male offspring increased from 0 to 100% as the parental rearing temperature increased from 30 °C to 34 °C (Table 1).
| Exposure temperature of F1parents ( °C) | Total offspring | Total number of females | Total number of males | Percentage of females | Percentage of males |
|---|---|---|---|---|---|
| 26 | 129 | 129 | 0 | 100 | 0 |
| 30 | 35 | 35 | 0 | 100 | 0 |
| 31 | 29 | 26 | 3 | 90 | 10 |
| 32 | 51 | 50 | 1 | 98 | 2 |
| 33 | 46 | 22 | 14 | 61 | 39 |
| 34 | 28 | 0 | 28 | 0 | 100 |
| 36 | 73 | 0 | 73 | 0 | 100 |
Critical time period for sex determination
For the first critical period test, the F1 eggs that were differentially exposed to 36 °C for 2-day intervals (labeled as 1–7) produced 47, 48, 29, 41, 39, 49, and 27 females, respectively, and 0 males. This indicated that 2 h of exposure to high temperature was not sufficient to kill the Wolbachia and yield male offspring. In the second test, the offspring of parents reared at constant 36 °C was 100% male (Table 2). The offspring of parents reared at 36 °C for the first half and 26 °C for the second half of their developmental time was 76% female and 24% male. The offspring of parents reared at 26 °C for the first half and 36 °C for the second half of their developmental time was 100% male. Finally, parents reared at constant 26 °C produced 100% daughters (Table 2).
| Group | Total Offspring | Total number of females | Total number of males | Percentage of females | Percentage of males |
|---|---|---|---|---|---|
| A: Constant 36 °C | 26 | 0 | 26 | 0 | 100 |
| B: 36 °C then 26 °C | 44 | 34 | 11 | 76 | 24 |
| C: 26 °C then 36 °C | 20 | 0 | 20 | 0 | 100 |
| D: Constant 26 °C | 25 | 25 | 0 | 100 | 0 |
Notes:
In groups B and C, the F1 generation was reared for the first half of their developmental time at the first temperature, and for its second half at the second temperature. The offspring (F2 generation) were reared at 26 °C in all four groups.
Wolbachia detection
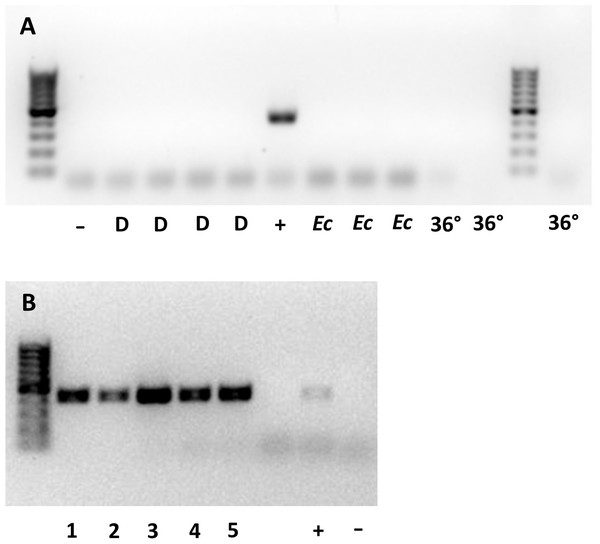
Whether they were sourced from 36 °C, a continuous supply of host eggs, or E. conspersus eggs (alternate host), all males tested negative for Wolbachia. None of the 10 gel electrophoresis lanes containing samples from males showed a band of the same size as the positive control for Wolbachia (Fig. 1A). The lanes with females all showed bands at the Wolbachia positive control position (Fig. 1B).
Figure 1: Gel plates testing for Wolbachia 16S rRNA gene in (A) male and (B) female Ooencyrtus mirus.
D = depleted Wolbachia (males from females reared with constant supply of host eggs for more than two weeks); −= negative control; + = positive control; Ec = males reared on Euschistus conspersus host eggs, 36° = males reared at 36 °C for two generations. The numbers at the bottom of (B) represent unique samples, each containing 3 O. mirus females.Intersexuality
For each trait examined, all males (69 individuals) had male characters and all females (28 individuals) had female characters; no intersexuality was observed.
Mating behavior
The most common interaction was for a male to approach a female head-on and drum his antennae on or under the female’s antennae. Within less than a second or up to 6 s, the female turned and walked away. Sometimes the male would chase the female as she walked away. On one occasion, when two males were pursuing the same female, one male tried to push the other away. In another observation, a male rocked side-to-side while antennating the female. Yet another time, two males antennated each other. Sometimes a male would pursue a female and drum his antennae on her abdomen from behind.
The newly emerged males had shorter and less frequent encounters with females than the older males did. On two different occasions, a male attempted to rear-mount a female. Nine different times, a male was observed touching the tip of his abdomen to the tip of the female abdomen for a second or two, at a 180° angle to the female with his head pointed away from her. After one of these times, the male moved backward quickly, antennae facing downward and still, and then stood in place and twitched for a few seconds, followed by grooming. The behavior preceding grooming was in stark contrast to usual male behavior, in which the males move forward, antennae facing forward and drumming rapidly.
Discussion
Effect of temperature on sex ratio
This study suggests that Wolbachia is present and active in O. mirus at temperatures below 34 °C. Based on the increase in males from 31−33 °C, the Wolbachia started to die off, or were impaired in their ability to manipulate the sex of the parasitoid offspring at these temperatures. The Wolbachia did not survive at 34 °C and higher. Ooencyrtus mirus thus tolerates higher temperatures than its symbiotic Wolbachia. Our sex ratio results differ from those for Ooencyrtus submetallicus (Howard) in Florida, USA, in which non-mated O. submetallicus produced males (Buschman & Whitcomb, 1980). However, our results parallel those of Wilson & Woolcock (1960) for O. submetallicus sourced from Trinidad Island in the Caribbean Sea, except that the critical temperature for Wolbachia in O. mirus is much higher. In O. submetallicus, the second generation was 100% female at 28 °C and 100% male at 29.4 °C. Ooencyrtus mirus had a wider transition range, with both sexes of offspring from mothers reared at 31−33 °C, and 34 °C was the lowest temperature that produced 100% males. As with O. mirus, the O. submetallicus parent generation reared at the high temperature was still mostly, or all female; only the offspring of that generation were male. The O. mirus - Wolbachia relationship is more similar to that of Ooencyrtus pityocampae (Merc.) in Israel. The offspring of O. pityocampae larvae kept at constant temperatures through 32 °C were female, with both male and female offspring arising from larvae kept at 32.5−33 °C, and only male offspring emerging from larvae kept at 34 °C or higher (Halperin, 1990). Likewise, for Trichogramma deion (misidentified as T. semifumatum (Perkins) (Hymenoptera: Trichogrammatidae) see (Pinto, 1999), but at lower temperatures, the offspring were almost all female when the parents were reared below 25.6 °C, but 97% sterile males and 3% intersexual individuals when the parents were reared at 32.2 °C (Bowen & Stern, 1966). Thelytokous Trichogramma pretiosum Riley have 1% male offspring at 28.26 °C and 40% males at 31 °C, whereas thelytokous Trichogramma cordubensis Vargas & Cabello produce 29% males at 28.26 °C and 100% males at 31 °C (Pintureauand & Bolland, 2001).
In summary, heat-killing of Wolbachia can occur in many hymenopteran species, but the minimum lethal temperature varies among Wolbachia in different host species. The minimum lethal temperature for Wolbachia in O. mirus is among the highest among those found in the literature. For all the aforementioned species, the temperatures at which F1 females are exposed during their immature development determines the sex of her F2 offspring, with high temperature leading to males and moderate temperature for each species leading to females. This implies that the eggs start to form even while the parent is a larva or pupa itself. In spite of this, however, O. mirus does not start ovipositing immediately after eclosion, and does not reach full oviposition capacity until 3 days after eclosion, even though the eggs laid before 3 days of age have a similar survival to eggs from parents aged 3 days and older (Power et al., 2021).
Critical time period for sex determination
The critical time period of exposing developing O. mirus females to high temperature that results in males appears to be more than two days in duration, as seen by the first critical period test producing all female offspring. The critical period also seems to be toward the middle or end of the developmental time, since all of the F2 offspring of parents subjected to 36 °C in the second half of development were male, but only about 1/3 of the offspring of parents subjected to 36 °C in the first half of development was male. Although beyond the scope of this study, more tests with varying times at 36 °C could further narrow down the critical period. Bowen & Stern (1966), for example, narrowed down the critical period for T. deion to the pupal stage of the parent. The need for more than two days of high temperature to induce production of males may explain how O. mirus can survive (i.e., not become 100% male) in the hot climate in Pakistan. Even in the hottest months, the average low temperatures are below 29 °C (Weather Spark, Excelsior, MN, USA). However, the average daytime high has a summer peak at 41 °C, so O. mirus may go dormant or seek out cooler microclimates during that time, unless they can endure short periods at such high temperatures. They die at a constant temperature of 38 °C in the lab (Power, Ganjisaffar & Perring, 2020a).
Halperin (1990) took the temperature transfer a step further with O. pitycampae. Females reared at 34 °C were transferred to 30 °C for oviposition, presumably over multiple days. First males emerged, then both males and females, and then females only. Laraichi (1978) did a similar study on Ooencyrtus fecundus Ferriere & Voegele. Like O. mirus, O. fecundus is an egg parasitoid of a pentatomid, in this case Aelia cognata Fieber. Parents were reared at 30 °C or 35 °C, and then switched to the opposite temperature after emergence. The rearing temperature determined the offspring sex for the eggs laid by the parent during her first three days of oviposition. After that, the opposite sex offspring appeared in increasing proportions. As the O. mirus in our study were given access to eggs for only one 24-hour period, it remains to be determined if the F2 sex ratio would stay the same if the females had access to eggs on subsequent days. In O. kuvanae, the opposite temperature effect was seen by Kamay (1976). Initial exposure to 35 °C, followed by rearing at 24 °C resulted in only 21% males, compared to 45% males reared at constant 24 °C. The effect was within the generation exposed to high temperature, not on the offspring. In this case, a different physiological mechanism than the suppression of Wolbachia appears to be at work.
As occurs in Trichogramma, the Wolbachia likely makes haploid eggs female by inhibiting chromosome separation during anaphase of the first round of mitosis (Stouthamer & Kazmer, 1994). Ooencyrtus mirus may have evolved from an arrhenotokous species that shifted to thelytoky because Wolbachia induced greater production of female offspring than were produced by non-infected O. mirus (Doremus & Hunter, 2020). In the current study, removal of Wolbachia did not enable O. mirus to return to arrhenotoky; rather it resulted in all males and thus an end to reproduction. Wolbachia is thus an obligate symbiont for O. mirus.
Wolbachia detection
The absence of Wolbachia in the male offspring was as expected. Ooencyrtus mirus likely evolved from an arrhenotokous haplo-diploid species wherein unfertilized eggs became male and fertilized eggs became female. Wolbachia makes the haploid eggs become female. That, in turn, creates selection pressure favoring Wolbachia-infected individuals because females do not need to expend energy on finding mates.
In an earlier study to determine the physiological host range of O. mirus at 26 °C, E. conspersus was the only host that produced males (Power, Ganjisaffar & Perring, 2020b). Further investigation would be needed to determine whether this was by chance or if E. conspersus inhibits Wolbachia in some way.
Intersexes
Out of 816 O. submetallicus, the first through fifth generation offspring reared at 21.1, 26.7, and 28.1 ° C were less than 1% males and no intersexes (Wilson & Woolcock, 1960). At 29.4 °C, however, there were 20 intersexes along with 78 males and 18 females in the second through fifth generations. In contrast, O. mirus had no intersexes out of 41 males and 28 male–female pairs; however our experimental set up may not have been optimal for the production of intersexes. We allowed newly emerged females to oviposit for only one day, whereas in some species infected with Wolbachia, intersexes are not produced on the first day of oviposition and increase in number in offspring produced in subsequent days (Tulgetske & Stouthamer, 2012).
Mating behavior
These are the first observations of mating behavior in O. mirus. Compared to O. kuvanae, an arrhenotokous species, O. mirus males typically undergo only the first step of the courtship ritual, engaging his antennae with the female’s antennae. The timing of the antennation is short in O. mirus, usually 0–4 s and maximum 6 s before the female turns and walks away. In O. kuvanae, antennation lasts 4–23 s. The antennation itself is different in that O. kuvanae male antennae surround and lock the female antennae. In response, the female’s antennae drop and point downward. After leg strikes by the male, the female goes into a “trance” (Ablard et al., 2011). In O. mirus, the antennae of both sexes keep moving and no trance is induced. Earlier observations on the mating behavior of O. kuvanae by Alzofon (1986) included the male contacting the female with his antennae, and then walking around her until they were facing each other and touching antennae. The female would turn side-ways and the male had to follow her, remaining head-to-head, or she would walk away. In O. kuvanae, the male mounts the female from behind while still facing forward. In O. mirus, the male sometimes went tail-to-tail with the female for a few seconds. If O. mirus males are as quick as Trichogramma evanescens Westwood (Hymenoptera: Trichogrammatidae), sperm may have been transferred. In T. evanescens, the mean duration for the first time a male copulates is under 2 s, and the mean is under 3 s for the next seven copulations (Damiens & Boivin, 2005). In the present study, the unusual behavior of one O. mirus male (i.e., orienting tail to tail with a female, quickly backing towards her, moving antennae downward and motionless, staying still, twitching for a few seconds, and grooming) may indicate that copulation occurred. The grooming behavior resembles that of Cephalonomia tarsalis (Ashmead) (Hymenoptera: Bethylidae). In this species, after the male dismounts, the two wasps separate from each other immediately, and then begin grooming (Cheng et al., 2004). In the parasitoid Cotesia urabae Austin & Allen (Hymenoptera: Braconidae), copulation is usually followed by stationary or grooming behavior (Avila, Withers & Holwell, 2017). Further study on O. mirus could show whether it is still capable of reverting to arrhenotoky, as shown in four Trichogramma spp. (Stouthamer, Luck & Hamilton, 1990), or whether, as in most natural populations having parthenogenesis-inducing Wolbachia, sexual reproduction is no longer possible (Stouthamer et al., 2010).
Conclusions
The increase in the male: female sex ratio of O. mirus offspring from parents reared at high temperatures (34 °C and 36 °C), combined with the presence of Wolbachia in females but not in males, strongly suggests that the thelytoky in O. mirus is due to reproductive manipulation by Wolbachia. Our experiments indicate that this manipulation occurs during the second half of the parent wasp immature development. Unlike some other parasitoid species, the loss of Wolbachia does not cause the production of intersexes in O. mirus, at least in the first exposure of a naïve female to host eggs.
Although O. mirus develops most quickly at 36 °C (Power, Ganjisaffar & Perring, 2020a), the current study indicates that above 30 °C, the male: female ratio increases due to the depletion of Wolbachia. Thus, the best temperature for maximizing females in a mass rearing and release program appears to be 30 °C. Future studies are needed to ensure that the offspring continue to be mostly female in successive oviposition days and subsequent generations at this temperature.