A new species and new records species of Pluteus from Xinjiang Uygur Autonomous Region, China
- Published
- Accepted
- Received
- Academic Editor
- Vladimir Uversky
- Subject Areas
- Biodiversity, Molecular Biology, Mycology, Taxonomy
- Keywords
- Distribution, New species, A new record, Phylogenetic analysis, Taxonomy
- Copyright
- © 2022 Qi et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. A new species and new records species of Pluteus from Xinjiang Uygur Autonomous Region, China. PeerJ 10:e14298 https://doi.org/10.7717/peerj.14298
Abstract
Xinjiang Uyghur Autonomous Region in China embraces a unique geographical and ecological environment, and the macrofungi represent a rich resource. However, few studies on the genus Pluteus have been reported from Xinjiang. In 2021, the macrofungal resources in Xinjiang were surveyed, and 10 specimens belonging to the genus Pluteus were collected. Based on the morphological study and molecular analysis, three species were recognized, P. aletaiensis, P. brunneidiscus, and P. hongoi. Pluteus aletaiensis is proposed as a new species. It is characterized by its bright yellow lamellae and stipe, brittle texture, subfusiform to vesicular pleurocystidia, with short pedicels to broadly lageniform to obtuse at apices, a hymeniderm pileipellis, containing dark brown intracellular pigment, and it grows on the ground. Pluteus brunneidiscus, a new record to China, is characterized by uneven, smooth, grayish brown to brown pileus, with an entire margin, and pointed or flatter apices intermediate cystidia, without apical hooks. Pluteus hongoi, a new record to Xinjiang Uyghur Autonomous Region, China, is characterized by the apical hook’s structure (commonly bifid) of pleurocystidia. The nuclear internal transcribed spacer (nrITS) and translation elongation factor 1-alpha (TEF1-a) region were used for the molecular analysis. Phylogenetic trees were constructed using both the maximum likelihood analysis (ML) and Bayesian inference (BI). Detailed descriptions of the three species are presented herein. Finally, a key to the list of eight species of the genus Pluteus knew from Xinjiang is provided.
Introduction
Genus Pluteus (Fr.) Quél. was established by Fries in 1836 and belongs to Basidiomycota, Hymenomycetes, Agaricales, Pluteaceae. The genus is distinguished from other agarics by the main features of the free and white, pink or pinkish brown lamellae, a pink spores print, mostly grow on rotten wood; smooth, non-dextrinoid, inamyloid, and pale pink basidiospores, divergent lamellar trama, thick- or thin-walled pleurocystidia (Vellinga & Schreurs, 1985; Singer, 1986; Justo et al., 2011a, 2011b).
Early studies (Lange, 1917; Imai, 1938; Singer, 1956), based on features such as pleurocystidia and pileipellis, divided the genus Pluteus into three sections: (1) section Pluteus Fr was characterized by pileipellis a cutis and thick-walled pleurocystidia, (2) section Hispidoderma Fayod was characterized by pileipellis a trichoderm composed of elongated cells and thin-walled pleurocystidia, and (3) section Celluloderma Fayod was characterized by pileipellis a hymeniderm or hymeniderm with cystidioid elements that consists of clavate to spheropedunculate cells and thin-walled pleurocystidia. However, other taxonomists held different perspectives. Kühner (1926, 1980) divided the genus Pluteus into two sections, one section with pileipellis a cutis and thick-walled pleurocystidia, and the other section with thin-walled pleurocystidia; Vellinga & Schreurs (1985), based on the characteristics of the pileipellis and pleurocystidia, divided the genus Pluteus into three sections: section Pluteus, section Villosi Vellinga & Schreurs, and section Celluloderma. Section Pluteus with pileipellis as a cutis and thick-walled pleurocystidia; section Villosi with pileipellis as a trichoderm and thin-walled pleurocystidia; section Celluloderma with a hymeniderm pileipellis and thin-walled pleurocystidia. In later studies, Singer (1958, 1986) divided section Celluloderma into two subsections based on the shape of pileipellis. (1) Subsection Mixtini Singer characterized by pileipellis as a hymeniderm with cystidioid elements that consists of two types of cells (ellipsoidal, balloon-shaped to inverted pyriform cells as well as elongated cells); (2) subsection Eucellulodermini Singer characterized by pileipellis as a hymeniderm that consists of cystidia-like cells. In addition, later Kobayashi (2002) established a new section-section Horridus S. Ito & S. Imai, including one species, P. horridilamellus S. Ito & S. Imai. Recently, based on the analyses of combined nSSU, ITS, and nLSU datasets, the genus Pluteus has been subdivided into three major lineages: (1) section Pluteus contains taxa with metuloid pleurocystidia and a pileipellis as a cutis, (2) section Hispidoderma are characterized by a pileipellis composed of elongated elements, very variable in shape and size, organized as a hymeniderm or trichoderm. (3) section Celluloderma includes species with non-metuloid pleurocystidia and a pileipellis as an euhymeniderm or an epithelioid hymeniderm composed of short elements, intermixed or not with elongate cystidioid elements and species with a cutis-like pileipellis and non-metuloid cystidia. The species with cutis-like pileipellis and possessing a partial veil, formerly placed in the genus Chamaeota (W.G. Sm.) Earle, are also included in Pluteus section Celluloderma (Justo et al., 2011b).
According to reports, about 300 species of the genus Pluteus were reported worldwide (Kirk et al., 2008), but only about 50 species were reported from China, distributed in 25 provinces or autonomous regions (Xu, 2016; Yang & Bau, 2010). In China, Patouillard (1893) was the first to report Pluteus species, then Chow (1935) was the first Chinese to studies on the genus Pluteus in China. Bi, Zheng & Li (1993) recorded 10 species of the genus in The macrofungus flora of China’s Guangdong Province; Redhead & Liu (1982) wrote a new species of p. luteus (Redhead & Liu, 1982) Redhead from China, now a synonym of p. variabilicolor Babos (Ševčíková & Dima, 2021). Xie, Wang & Wang (1986) recorded seven species of the genus Pluteus in the Changbai Mountain area in Illustrations of Agarics from Changbai Mountains, Mao (2000) recognized 12 species of genus Pluteus in China in the Macrofungi in China, Li & Bau (2003) reported five species of genus Pluteus in Changbai Mountain area in Mushrooms of Changbai mountain in China; Zhuang et al. (2005) included 15 species of genus Pluteus in Fungi of Northwestern China. In recent years, many new and newly recorded species in China have also been published (Xi et al., 2011; Hosen et al., 2018, 2019, 2021).
Although the Xinjiang Uyghur Autonomous Region has a unique geographical and ecological environment, only five Pluteus species have been reported from Xinjiang, and many resources need to be clarified. P. thomsonii (Berk. & Broome) Dennis. was reported from the Jengish Chokusu (Mao, Wen & Sun, 1978). Wang & Zhao (1997) reported P. cervinus (Schaeff.) P. Kumm. from the Central Tianshan Forest Region. Mao (2000) recorded P. umbrosus (Pers.) P. Kumm. from Xinjiang. Zhao (2001) recognized P. cervinus, P. leoninus (Schaeff.) P. Kumm., and P. pellitus (Pers.) P. Kumm. from Xinjiang.
We conducted a preliminary investigation of the taxonomy of Pluteus species in Xinjiang. The goal of the present study was to provide an annotated list of all species recorded, describe one new species, list newly recorded species from China and newly recorded species from Xinjiang, give detailed descriptions and illustrations of three species, and clarify the phylogenetic relationships of the revealed species and related taxa from the genus Pluteus based on morphological and molecular studies.
Materials and Methods
Specimens and morphological description
Site description
Xinjiang Uygur Autonomous Region is located in the hinterland of the Eurasian continent of northwestern China. For example, there are distinctive landforms, including the world’s second-highest peak. It is surrounded by high mountains, resulting in early dryness and precipitation varies significantly among regions. There are continental cold temperate zone cold climate, temperate zone continental monsoon climate, temperate zone continental arid climate, and alpine climates from north to south (Yao et al., 2022).
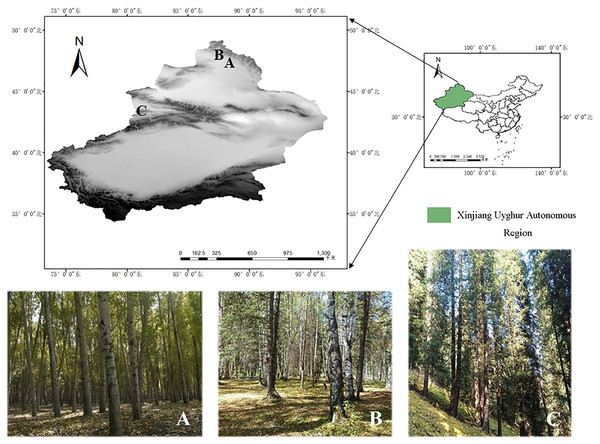
In this study, the specimens of the genus Pluteus were collected from Kelan River Valley in Altay City (47°50′14.66″N, 88°13′23.41″E). Altay Region with an altitude of about 577 m, Wolong Bay in Kanas (48°65′77.01″N, 87°03′82.11"E). Altay Region with an altitude of 1,333 m, and Shuimogou in Regiment 74 (43°12′50.69″N, 80°13′18.44″E). Zhaosu County, Ili Kazakh Autonomous Prefecture with an altitude of 1,764 m. See Fig. 1 for details.
Figure 1: Specific collection sites for three species.
(A) Habitat/landscape for Pluteus aletaiensis. (B) Habitat/landscape for P. hongoi. (C) Habitat/landscape for P. brunneidiscus.Collection and morphological studies
Photos of fresh basidiomata were taken in the field, scientifically fully reflecting the growth environment and the characters, including the shape of the pileus, the color of lamellae, and color codes followed by Munsell Soil Color Charts (Munsell, 2009). The size of basidiomata was measured when fresh, and detailedly recorded the macroscopic characteristics, such as shape, size, and color of the pileus, odor, color, and density of lamellae. A small portion of fresh context and lamellae was dried on silica gel and used for DNA extraction. Fresh basidiomata were dried at 40–50 °C using an electric drier and preserved at the Herbarium of Mycology of Jilin Agricultural University (HMJAU). The observation of microstructure characteristics were based on dry specimens. The dry specimens were rehydrated in 94% ethanol for microscopic examination and then mounted in 3% potassium hydroxide (KOH), 1% Congo Red, and Melzer’s Reagent, using a light microscope (ZEISS, DM1000, Oberkochen, Germany). The morphological descriptions follow Largent, Johnson & Watling (1977). As previously described in Rao et al. (2021b), data were collected. Specifically, the following symbols were used in the description: [n/m/p] indicates that ‘n’ randomly selected basidiospores from ‘m’ basidiomata of ‘p’ collections were measured, ‘avl’ means the average length of basidiospores, except for the extreme values, ‘avw’ refers to the average width of the basidiospores, except the extreme values, ‘Q’ represents the quotient of the length and width of a single basidiospore inside view, ‘Qm’ refers to the average Q value of all basidiospores ± standard deviation. Dimensions for basidiospores are given as (a)b–c(d). The range of b–c contains a minimum of 90% of the measured values. Extreme values (i.e., a and b) are given in parentheses.
Research methods of molecular systematics
DNA extraction, PCR amplification, and sequencing
The total DNA of the specimens was extracted by the new plant genomic DNA extraction kit from Jiangsu Kangwei Century Biotechnology Limited Company. The amplification primers of the ITS nrDNA (ITS) regions were ITS1 and ITS4 (White et al., 1990), and TEF1-α regions were EF1- 983F and EF1-1567R (Rehner & Buckley, 2005). The amplification reactions were carried out in a 25 μl system. The total amount of PCR mixed was as follows: dd H2O 13.5 μl, 10 × Taq Buffer 5 μl, 10 mM dNTPs 1 μl, 10 mM upstream primer 1 μl, 10 mM downstream primer 1 μl, DNA sample 2 μl, 2 U/μm Taq Polymerase 1.5 μl. The cycle parameters were as follows: 4 min at 94 °C; 30 s at 94 °C, 40 s at 53 °C, 1 min at 72 °C for 36 cycles; 10 min at 72 °C; storage at 4 °C (Xu, 2016). The PCR product was subjected to 1% agarose gel electrophoresis. The purified PCR products were sent to Sangon Biotech Limited Company (Shanghai, China) for sequencing using the Sanger method. The sequencing results were clipped with Seqman 7.1.0 (Swindell & Plasterer, 1997) and then submitted to GenBank (https://www.ncbi.nlm.nih.gov/genbank/).
Data analysis
Based on BLAST results, morphological similarities, and the related articles (Menolli, Asai & Capelari, 2010; Justo et al., 2014; Menolli, Justo & Capelari, 2015; Rao et al., 2021a; Ševčíková et al., 2022), ITS and TEF1-α sequences obtained and related to these samples are listed in Table 1. The ITS and TEF1-α dataset comprised 106 representative sequences showing the highest similarity to Pluteus spp., and two sequences of Volvopluteus michiganensis (A.H. Sm.) Justo & Minnis. as an outgroup.
| Taxon | Collection | Country | nrITS | TEF1α |
|---|---|---|---|---|
| pluteus alniphilus | – | France | KJ009677 | – |
| p. alniphilus | – | Russia | KJ009676 | – |
| P. aff. romellii | AJ 215 | Spain | HM562054 | ON813269 |
| P. aff. romellii | BRNM 792987 | Czech Republic | ON864083 | ON813272 |
| P. aff. romellii | LB 15121104 | Spain | ON864082 | ON813271 |
| P. aff. romellii | LE 312975 | Russia | ON864081 | ON813270 |
| P. aff. romellii | LE 313340 | Russia | ON864084 | ON813273 |
| P. aurantiorugosus | GDGM41547 | China | MK791275 | – |
| P. aurantiorugosus | GM 2580 | Spain | ON864101 | – |
| P. aurantiorugosus | LE 312803 | Russia | ON864105 | – |
| P. aurantiorugosus | LE 312815 | Russia | ON864103 | ON813296 |
| P. aurantiorugosus | LE 313555 | Russia | ON864104 | – |
| P. aurantiorugosus | Voucher 880 | Italy | JF908608 | – |
| P. aurantiorugosus | Voucher 2847 | Italy | JF908613 | – |
| P. aureovenatus | SP 393697 | Brazil | FJ816663 | KJ010056 |
| P. aureovenatus | SP 394388 | Brazil | HM562160 | – |
| P. aureovenatus | SP 416735 | Brazil | KM983702 | – |
| P. austrofulvus | AJ 857 | USA | KM983701 | ON813290 |
| P. austrofulvus | AJ 860 | USA | KM983699 | ON813288 |
| P. austrofulvus | iNaturalist 112016967 | USA | ON864095 | ON813291 |
| P. austrofulvus | iNaturalist 112219822 | USA | ON864096 | ON813292 |
| P. austrofulvus | iNaturalist 112280046 | USA | ON864097 | ON813293 |
| p. aletaiensis | HMJAU 60207 | China | OM991943 | OP573273 |
| p. aletaiensis | HMJAU 60208 | China | OM992247 | OP573274 |
| p. aletaiensis | HMJAU 60209 | China | OM992249 | OP573275 |
| p. brunneidiscus | – | Canada | KJ009691 | – |
| p. brunneidiscus | – | Canada | KJ009692 | – |
| p. brunneidiscus | HMJAU 60206 | China | OM991893 | – |
| p. brunneidiscus | HMJAU 60210 | China | OM943513 | – |
| P. castaneorugosus | LE 313071 | Vietnam | MT611237 | – |
| p. cervinus | – | Russia | KJ009629 | – |
| p. cervinus | – | Russia | KJ009632 | – |
| P. exilis | – | USA | KJ009659 | – |
| P. fulvibadius | AJ 815 | USA | KM983698 | ON813285 |
| P. fulvibadius | HRL3391 | Canada | ON864094 | ON813287 |
| P. fulvibadius | MO 270623 | USA | ON864093 | ON813286 |
| P. globiger | ICN139025 | Brazil | JQ065030 | – |
| p. hongoi | – | Japan | HM562100 | – |
| p. hongoi | – | Japan | HM562101 | – |
| p. hongoi | HMJAU 60205 | China | OM302007 | – |
| P. iguazuensis | NK I10 | Brazil | KM983704 | – |
| p. kovalenkoi | – | Russia | KJ009697 | – |
| P. pallescens | K (M) 93678 | UK | ON864073 | – |
| P. parvicarpus | LE 313357 | Russia | ON864114 | ON813302 |
| P. parvicarpus | LE 313631 | Russia | ON864115 | ON813303 |
| P. parvisporus | AJ 855 | USA | ON864099 | ON813295 |
| P. parvisporus | iNaturalist 112236342 | USA | ON864098 | ON813294 |
| P. paucicystidiatus | SP 394383 | Brazil | HM562173 | – |
| P. pauperculus | JAC11068 | New Zealand | MN738636 | – |
| P. pauperculus | JAC9790 | New Zealand | MN738621 | – |
| P. phlebophorus | AJ81 | Spain | HM562039 | ON133554 |
| P. romellii | AJ 232 | Spain | HM562062 | ON813280 |
| P. romellii | BRNM 761731 | Czech Republic | ON864065 | ON813278 |
| P. romellii | BRNM 816205 | Czech Republic | ON864063 | ON813276 |
| P. romellii | BRNM 817530 | Slovakia | ON864072 | ON813282 |
| P. romellii f. albidus | MCVE 28336 | Italy | KM035790 | – |
| P. romellii var. luteoalbus | BRNM 788199 | Czech Republic | LT838190 | – |
| P. rugosidiscus | BRNM761706 | Slovakia | MH010876 | LT991752 |
| P. rangifer | – | Russia | KJ009651 | – |
| P. rangifer | – | Russia | KJ009654 | – |
| p. salicinus | – | Spain | HM562051 | – |
| p. salicinus | – | Spain | HM562174 | – |
| p. saupei | – | USA | HM562113 | – |
| p. shikae | – | Japan | HM562095 | – |
| p. shikae | – | Russia | KJ009696 | – |
| P. siccus | LE 313356 | Russia | ON864113 | ON813301 |
| P. stenotrichus | AJ 352 | Dominican Republic | JN603201 | – |
| P. sternbergii | PRM 154258 | Czech Republic | ON864116 | – |
| P. sublaevigatus | SP 393694 | Brazil | FJ816667 | – |
| Pluteus sp. | AJ 842 | Dominican Republic | KM983705 | – |
| Pluteus sp. | SP 416739 | Brazil | KM983703 | – |
| P. vellingae | BRNM 817769 | Czech Republic | ON864108 | ON813297 |
| P. vellingae | ECV 3201 | USA | AY854065 | AY883433 |
| P. vellingae | FG02092019008 | Slovenia | ON864112 | ON813299 |
| P. vellingae | GM 3260 | Spain | ON864107 | ON813298 |
| P. vellingae | OKA-TR512 | Turkey | – | ON813300 |
| Volvopluteus michiganensis | – | China | MW242664 | – |
| V. michiganensis | – | China | MW242665 | – |
Note:
Bold fonts are the sequences to be determined in this study.
For the ITS dataset, the ‘auto’ strategy and normal alignment mode of MACSE V2.03 (Ranwez et al., 2018) and MAFFT (Katoh & Standley, 2013) were used for sequence alignment, then manually adjusted in BioEdit v7.1.3 (Hall, 1999). ModelFinder (Kalyaanamoorthy et al., 2017) selected the best-fit models using the Bayesian information criterion (BIC). The maximum likelihood (ML) analyses were performed in IQTree 1.6.8 (Nguyen et al., 2015), and the Bayesian inference phylogenies were performed in MrBayes 3.2.6 (Ronquist et al., 2012) (two parallel runs, 2,000,000 generations), in which the initial 25% of sampled data were discarded as burn-in. The above software was integrated into PhyloSuite 1.2.2 (Zhang et al., 2020). The ML phylogenetic tree was evaluated using the bootstrap method with a bootstrap value of 1,000 replicates; BI determined that the analysis reached smoothness with variance <0.01 and terminated the calculation. The evolutionary tree was followed up with Figtree v1.4.
For the ITS + TEF1-α dataset, sequence alignment was performed for ITS and TEF1-α using the "automatic" strategy of MACSE V2.03 (Ranwez et al., 2018) and MAFFT (Katoh & Standley, 2013) and normal alignment mode, respectively, and then manually adjusted in BioEdit v7.1.3 (Hall, 1999). Afterward, ITS and TEF1-α sequences were combined using PhylosuitV1.2.2 (Zhang et al., 2020). ModelFinder (Kalyaanamoorthy et al., 2017) selected the best-fit models using the Bayesian information criterion (BIC). The maximum likelihood (ML) analyses were performed in IQTree 1.6.8 (Nguyen et al., 2015), and the Bayesian inference phylogenies were performed in MrBayes 3.2.6 (Ronquist et al., 2012) (two parallel runs, 2,000,000 generations), in which the initial 25% of sampled data were discarded as burn-in. The above software was integrated into PhyloSuite 1.2.2 (Zhang et al., 2020). The ML phylogenetic tree was evaluated using the bootstrap method with a bootstrap value of 1,000 replicates; BI determined that the analysis reached smoothness with variance <0.01 and terminated the calculation. The evolutionary tree was followed up with Figtree v1.4.
Nomenclature
The electronic version in Portable Document Format (PDF) will represent a published work according to the International Code of Nomenclature for algae, fungi, and plants. Hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. In addition, new names contained in this work have been submitted to MycoBank from where they will be made available to the Global Names Index. The unique MycoBank number can be resolved and the associated information viewed through any standard web browser by appending the MycoBank number contained in this publication to the prefix “http://www.mycobank.org/MycoTaxo.aspx?Link=T&Rec=.”. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central, and CLOCKSS.
Results
Phylogenetic analyses
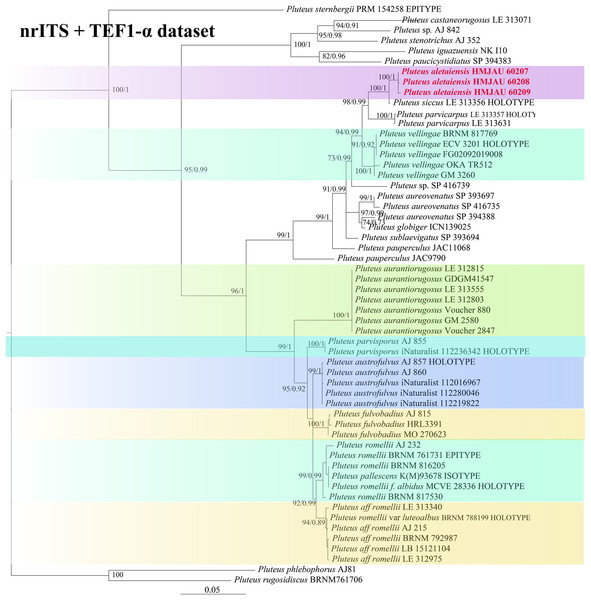
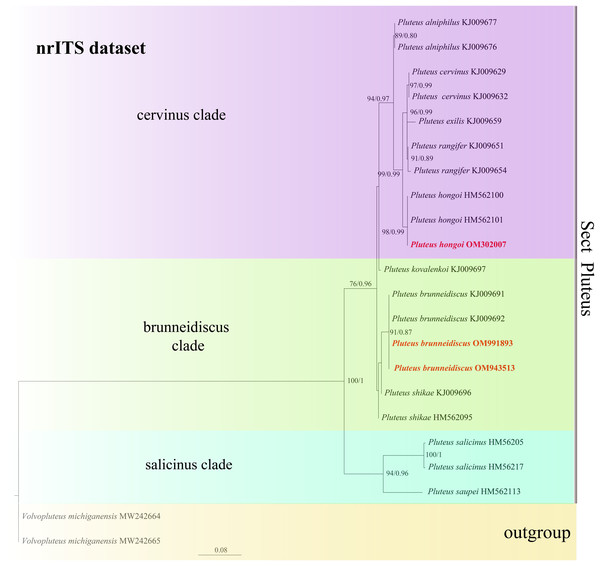
In the dataset, 111 sequences derived from two gene loci (ITS and TEF1-α) from 35 samples were used to build phylogenetic trees; nine of these were newly generated, with six ITS sequences and three TEF1-α sequences. The phylogenetic construction performed via ML and BI analysis for the two combined datasets showed a similar topology (Figs. 2 and 3).
Figure 2: Phylogenetic tree of the section Celluloderma of the genus Pluteus.
Best tree from the ML and BI analysis of the nrITS + TEF1-α dataset. The two values of internal nodes respectively represent maximum likelihood bootstrap (MLBP)/Bayesian posterior probability (BIPP). In at least two analyses, the thick node indicates the significantly-supported branch (MLBP ≥ 70, BIPP ≥ 95%). The collection number is marked after the species name. New species is indicated in bold and red font.Figure 3: Phylogenetic tree of the section Pluteus of the genus Pluteus.
Best tree from the ML and BI analysis of the nrITS dataset. The two values of internal nodes respectively represent maximum likelihood bootstrap (MLBP)/Bayesian posterior probability (BIPP). In at least two analyses, the thick node indicates the significantly-supported branch (MLBP ≥ 70, BIPP ≥ 95%). The GenBank accession number is marked after the species name. Two species from China are expressed in bold and red font, and Volvopluteus michiganensis is selected as the outgroup.In the present study, six sequences of P. aletaiensis were gathered into a single branch along with high support (100/1). Pluteus aletaiensis is classified into the section Celluloderma, and sisters to P. siccus E.F. Malysheva, P. parvicarpus E.F. Malysheva, P. vellingae Justo, Ferisin, Ševčíková, Kaygusuz, G. Muñoz, Lebeuf & S.D. Russell, P. sublaevigatus (Singer) Menolli & Capelari and P. globiger Singer,. with a very high support rate (Fig. 2). The sequence of P. brunneidiscus and P. hongoi from Xinjiang Uygur Autonomous Region were gathered into the sect. Pluteus and formed independent clades with high support (Fig. 3).
Taxonomy
Pluteus aletaiensis Z.X. QI, B. Zhang & Y. Li, sp. nov.
MycoBank No. MB 840297
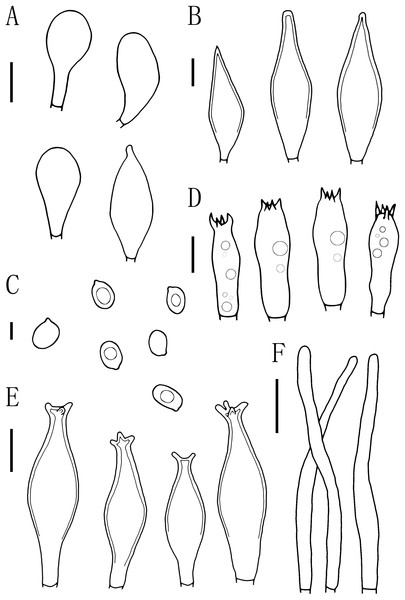
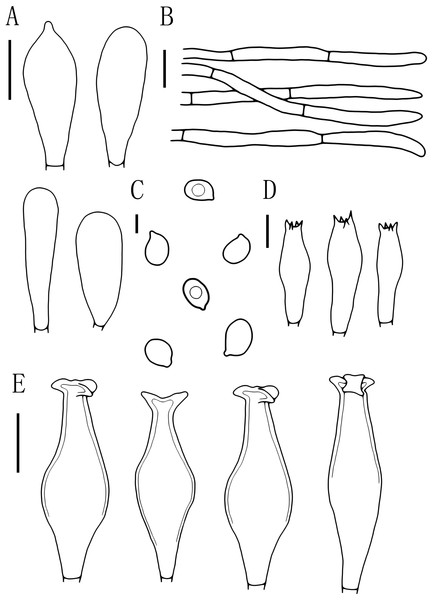
Figure 4: Basidiomata features.
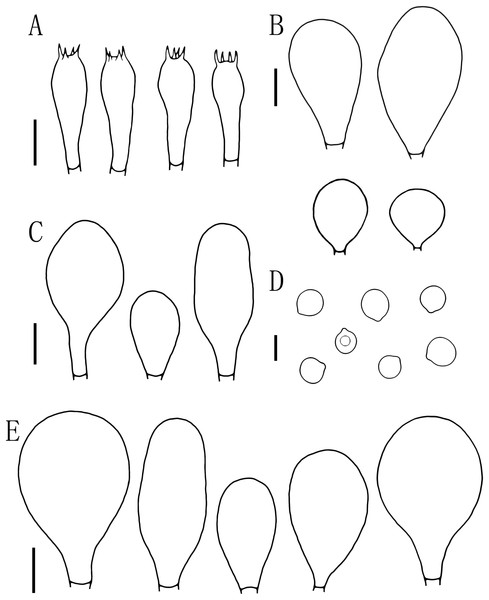
(A–E) Pluteus aletaiensis. (F–G) P. brunneidiscus. (H–I) P. hongoi. (A–I) Photos by Zheng-xiang Qi. Scale bars: 1 cm.Figure 5: Microscopic features of Pluteus aletaiensis.
(A) Basidia. (B) Pileipellis. (C) Cheilocystidia. (D) Basidiospores. (E) Pleurocystidia. Scale bars: (D) 5 µm; (A, B, C, E) 10 µm.Etymology. The epithet “Aletaiensis” refers to the Aletai Region, the location of the holotype.
Holotype. CHINA. Xinjiang Uygur Autonomous Region, Aletai Region, Aletai City, Cran River Valley, 47°50′14.66″N, 88°13′23.41″E, ASL 577 m, 14 September 2021, Z.X. Qi (HMJAU 60207), GenBank: OM992247.
Diagnosis. Pluteus aletaiensis differs from P. romellii by its bright brownish yellow, dark brownish-yellow in the middle of pileus, surface cover with white punctuations stipe, brittle texture. Microscopically, narrower pleuro- and cheilo-cystidia, a hymeniderm pileipellis, with globose to short-clavate, containing dark brown intracellular pigment.
Description
Basidiomata tiny to small size. Pileus 5–22.5 mm broad, initially paraboloid to hemispherical, later oblong hemispherical with an umbo, brownish-yellow when young (2.5YR 4/10), bright brownish yellow at maturity (7.5Y 6/12-7.5Y 8/14), dark brownish-yellow in the middle (7.5Y 5/8), not striated, with varying degrees of dehiscence at the margin at maturity. Context yellow, 0.5–1 mm thick. Lamellae close, bright yellow to yellowish (2.5Y 8/6-2.5Y 9/8), edges white (2.5Y 9/6), free, unequal, ventricose, 2–6 mm wide, entire. Stipe 4.5–25 mm long and 1.5–3 mm wide, central, slender, clavate, slightly thick and slightly curved at base, brittle, bright yellow to yellowish (2.5Y 8/6-2.5Y 9/8), with longitudinal striation, white punctuations, and white pruinose mycelium at the base. Spore print unknown.
Basidiospores [100, 12, 3] (5.5)6.0–7.5(8.0) × 5.5–6.5 (7) μm, avl = 7.0 μm, avw = 6.0 μm, Q = (1.08)1.09–1.20(1.24), Qm = 1.16 ± 0.05, globose, subglobose to broadly elliptical, slightly pinkish, thick-walled, smooth, non-dextrinoid, partially containing one droplet or irregular inclusions. Basidia 27–43 × 7–12(13) μm, fusiform to clavate, usually 4-sterigmate, 2–3 sterigmate occasional, thin-walled, and hyaline in KOH. Pleurocystidia 51–75(80) × (11)13–22(23) μm, abundance, subfusiform to vesicular, with short pedicels to broadly lageniform to obtuse at apices, thin-walled, and hyaline in KOH. Cheilocystidia 25–40 × (11)12–20 μm, abundant, clustered, vesiculose or pyriform to broadly fusoid-ventricose with pedicels short necks, with obtuse at apices, thin-walled, and hyaline in KOH. Pileipellis a hymeniderm, cells 15–43 × 10–34(35) μm, composed of globose to short-clavate cells, thin-walled, and hyaline in KOH, containing dark brown intracellular pigment, cells tightly adhering one to another. Stipitipellis a cutis of cylindrical hyphae, elements 5–17 μm wide, thin-walled, hyaline in KOH, septate, without apparent contents. Clamp connections absent in all tissues.
Ecology and distribution. Scattered on the ground in the broad-leaved forest (Populus alba var. pyramidalis Bge). Known from Xinjiang Uygur Autonomous Region of China.
Additional specimens examined. China. Xinjiang Uygur Autonomous Region, Aletai Region, Aletai City, Cran River Valley, 47°50′14.56″N, 88°13′23.45″E, ASL 580 m, 15 September 2021, Z.X. Qi, D.M. Wu, N. Gao & B.K. Cui, HMJAU 60208 (OM991943); Xinjiang Uygur Autonomous Region, Altay Region, Altay City, Cran River Valley, 47°50′14.69″N, 88°13′23.47″E, ASL 576 m, 16 September 2021, Z.X. Qi, D.M. Wu, N. Gao & B.K. Cui, HMJAU 60209 (OM992249).
Note. In our phylogenetic tree (Fig. 2), P. aletaiensis clustered with similar species of section Celulloderma and together with P. siccus P. parvicarpus, P. vellingae, P. sublaevigatus, and P. globiger with high support (Fig. 2). On phylogenetic trees, P. siccus display a close relationship to P. aletaiensis; morphologically, they are distinctly different, P. siccus (Ševčíková et al., 2022) is distinguishable from P. aletaiensis due to its slightly velvety pileus with a greenish hue, and smaller basidiospores (about 4.6–6.0 × (3.5–)4.5–5.5 µm), the polymorphic cheilocystidia, and grows on decaying wood and geographically distributed in the Russian Far East. P. parvicarpus (Ševčíková et al., 2022) could be distinguished from P. aletaiensis by its sulcate-striate at the margin of pileus, smaller basidiospores (about 4.5–6.0 × 4.2–5.5 µm), solitary on fallen branches of deciduous trees, and geographical distribution in the Russian Far East. P. aletaiensis and P. vellingae (Ševčíková et al., 2022) are both with yellow-brown to brown pileus and similar basidiospores. P. vellingae has broadly clavate to clavate or ovoid pleurocystidia, and it grows on coniferous or deciduous wood. P. sublaevigatus (Menolli, Asai & Capelari, 2010) differs from P. aletaiensis with its slightly rugulose at the center of the pileus, and translucently striate at the margin, free to subfree lamellae with lamellulae, and gregarious on decaying wood. P. aletaiensis and P. globiger (Singer & Digilio, 1952; Dias & Cortez, 2013) are both smaller pileus (diameter not more than 23 mm), but the lamellae of P. aletaiensis are bright yellow to yellowish, while P. globiger are greyish orange; on the other hand, P. globiger has ventricose of the cheilocystidia.
Morphologically, we excluded some relatively names species that are highly similar to P. aletaiensis, such as P. fulvibadius Murrill, P. romellii (Britzelm) Lapl., P. sternbergii Velenovský, and P. sulphureus Velenovský.
P. fulvibadius Murrill is also similar to P. aletaiensis. P. fulvibadius, which is widely distributed in the United States, has been reported by many mycologists (Murrill, 1917; Minnis, 2008; Minnis & Sundberg, 2010). P. fulvibadius differs from P. aletaiensis by its bigger pileus, which reaches 15–50 mm broad, slight to strongly rugose around the center, and translucently striate at the margin. In microfeatures, the pleurocystidia of P. fulvibadius are cylindrical-clavate; the pleurocystidia and the cheilocystidia are covered with an apical cap of amorphous mucilaginous material (Minnis, 2008; Minnis & Sundberg, 2010; Ševčíková et al., 2022).
P. romellii is easily confused with P. altaiensis due to its highly similar morphology, and a synonym of P. romellii is P. lutescens (Singer, 1956; Vellinga & Schreurs, 1985; Orton, 1986; Minnis, 2008). However, the pileus of P. aletaiensis is oblong hemispherical with an umbo, bright brownish yellow, dark brownish-yellow in the middle, while the color of P.romellii variable (date-brown, umber, or snuff-brown), glabrous, venation-like protrusions, and with translucent-striate at the margin; on the other hand, pleuro- and cheilo-cystidia of P. romellii are broader (52–62 × 14–30 μm and 23–61 × 12–36 μm respectively) than P. altaiensis (51–75(–80) × (11–)13–22(–23) μm and 25–40 × (11–)12–20 μm respectively) (Singer, 1956; Orton, 1986; Vellinga, 1990; Minnis, 2008). In the phylogenetic tree, P. romellii and P. altaiensis are not clustered in the same branch.
Ševčíková et al. obtained the molecular sequence of P. sternbergii (ON864116) and verified the position of P. sternbergii in the phylogenetic tree, not in the romellii clade, but in the cinereofuscus clade, therefore, selected collection PRM 154258 as the epitype of P. sternbergii. (Ševčíková et al., 2022). Morphologically, the basidiospores, basidia, pleurocystidia, and stipitipellis of P. aletaiensis and P. sternbergii are similar, while P. sternbergii grows in the stump of Populus.
For P. sulphureus, Ševčíková considers the specimens preserved in herbarium PRC (Velenovský no. 95!) as not belonging to the genus Pluteus (Ševčíková et al., 2022).
Pluteus brunneidiscus Murrill, N. Amer. Fl. (New York) 10(2): 131 (1917).
Syn.: Pluteus washingtonensis Murrill, N. Amer. Fl. (New York) 10(2): 135 (1917).
Figure 6: Microscopic features of Pluteus brunneidiscus.
(A) Cheilocystidia. (B) Intermediate cystidia. (C) Basidiospores. (D) Basidia. (E) Pleurocystidia. (F) Pileipellis. Scale bars: (C) 5 µm; (B, D) 10 µm; (A, E) 20 µm; (F) 40 µm.Description
Basidiomata large-sized. Pileus 68 mm broad, surface shiny and smooth, grayish brown to brownish brown (10.0YR 4/10-10.0YR 3/6), margin spreading to partially collapsed, uneven, entire, partly collapsed. Lamellae close, dirty white (10.0YR 9/2), turning dark brown at maturity (5.0YR 5/4), free, thick, unequal, slightly umbo. Stipe 79 mm × 8.5 mm, central, clavate, slightly wider at the base, fibrous, with longitudinal brownish (5.0YR 4/10) ciliate stripes, denser toward the base. Spore print unknown.
Basidiospores [60, 1, 1] (7.0)7.5–8.0(9.0) × 5.0–6.0 μm, avl = 8.0 μm, avw = 5.5 μm, Q = 1.16–1.50(1.60), Qm = 1.45 ± 0.10, broadly elliptical, oval to ovoid, slightly pinkish, slightly thick-walled, smooth, non-dextrinoid. Basidia 24–33 × 7–11(12) μm, clavate, 4-sterigmate, sterigmate short, less than 1 μm, thin-walled, and hyaline in KOH. Pleurocystidia 49–75(78) × (13)15–26 μm, mass, scattered, fusiform or narrowly fusiform to narrowly utriform, with 2–4 apical hooks (commonly entire), without small lateral hooks, hyaline, smooth, neck thickness up to 2–3 μm. Intermediate cystidia fusiform, poke-shaped, with pointed or flatter apices, without apical hooks, smaller than the pleurocystidia, thick-walled, hyaline. Cheilocystidia 38–60 × (11)12–15 μm, crowded, grouped to clustered, clavate or narrowly clavate to capitate, with obtuse at apices, thick-wall, and hyaline in KOH. Lamellar edge sterile. Pileipellis a cutis, with terminal elements 80–146 × 6–18 μm, individual elements cylindrical, some strongly tapering towards apex, mostly filled with brown intracellular pigment, thin-walled, smooth. Stipitipellis a cutis, hyphae 5–25 μm wide, cylindrical, hyaline or brown intracellular pigment, smooth, and thin-walled. Clamp connections absent in all tissues.
Ecology. Solitary or gregarious, growing on decayed wood (Betula, Umbellularia, Populus) or the humus layer under hardwoods or conifers in summer.
Distribution. Canada, Russia, USA, Turkey (Justo et al., 2014; Kaygusuz et al., 2021), and China (Xinjiang Uygur Autonomous Region)
Specimens examined. CHINA. Xinjiang Uygur Autonomous Region, Ili Kazakh Autonomous Prefecture, Zhaosu County, Shuimogou in Regiment 74, 43°12′50.69″N, 80°13′18.44″E, ASL 1764 m, 1 August 2021, Z.X. Qi, D.M. Wu, N. Gao & Y. Wang, HMJAU 60206 (OM991893, OM943513).
Note. Pluteus brunneidiscus and P. washingtonensis Murrill were first reported from USA (Murrill, 1917). However, Singer (1956), and Banerjee & Sundberg (1995) suggested that these two species may be the same. Singer (1986) cited P. washingtonensis as “probably conspecific with P. brunneidiscus” and with only one difference in the size of spores; besides, the spores of P. washingtonensis (about 6.5–9.6 × 5.3–7.1 µm) are slightly larger. Banerjee & Sundberg (1995) described the terminal elements on the pileipellis of P. brunneidiscus and P. washingtonensis as “versiform.” However, Justo & Castro (2007a) believe that the terminal elements were difficult to observe in the type collections. In modern collections, the shape of these elements was variable within the same basidiocarp and with the same range of variation observed. Therefore, they treated P. washingtonensis as synonymous with P. brunneidiscus. P. brunneidiscus in Europe was firstly described by Justo & Castro (2007a). In our study, P. brunneidiscus is reported as a new record in China.
In our phylogenetic analysis, P. brunneidiscus gathered into sect. Pluteus, with two other species—P. shikae Justo and E.F., P. kovalenkoi E.F.—in clade brunneidiscus. These three species can be distinguished from molecular data. Morphologically, many of their characters are rather variable, such as basidiospores and pleurocystidia with developed apical hooks. However, P. kovalenkoi can also be distinguished from P. brunneidiscus by the shape of pleurocystidia (Justo et al., 2014). We also compared other characters, as detailed in Table 2 (Justo et al., 2014; Kaygusuz et al., 2021).
| Pluteus brunneidiscus |
Pluteus brunneidiscus in China |
Pluteus shikae | Pluteus kovalenkoi | |
|---|---|---|---|---|
| Pileus | 30–70 mm, hemispherical or campanulate to convex or plano-convex, smooth or fibrillose, brown to pure white, margin striate |
68 mm, spreading to partially collapsed, shiny and smooth, grayish brown to brownish brown | 20–50 mm, hemispherical or campanulate to convex or plano-convex, smooth, brown, margin striate | 37–49 mm, obtusely campanulate to convex or plano-convex, smooth, fibrillose, brown or gray-brown, margin striate |
| Lamellae | crowded, free, ventricose, 7 mm broad, white to pink, flocculose edges | close, free, dirty white to dark brown, thick, unequal, ventricose | crowded, free, ventricose, 6 mm broad, white to pink, flocculose edges | crowded, free, slightly ventricose, 5 mm broad, white-cream to pink, concolorous edges |
| Stipe | 30–80 × 3–7 mm, cylindrical, white, smooth or with longitudinal brown or gray-brown fibrils | 79 × 8.5 mm, central, clavate, wider at the base, fibrous, with longitudinal brownish ciliate stripes | 30–65(–70) × 3–6 mm, cylindrical, white, smooth or with longitudinal brown or gray-brown fibrils | 70–80 × 5–6 mm, cylindrical, white or white-cream, glabrous or slightly pruinose |
| Context | white | not recorded | white | white |
| Spore print, Taste, Smell |
Taste similar to smell or indistinct, Spore print pink to pinkish brown. | not recorded | not recorded | not recorded |
| Basidiospores | 6.5–9.6(–10.5) × (4.5–)5.0–7.1 μm, slightly constricted in the middle | (7.0)7.5–8.0(9.0) × 5.0–6.0 μm, broadly elliptical, oval to ovoid,pinkish, smooth, non-dextrinoid | 5.5–8.0 × (3.5–)4.0–5.5(–6.0) μm, slightly constricted in the middle | (7.3–)7.6–9.0(–9.5) × 4.6–5.8 μm, constricted in the middle |
| Basidia | 15–28 × 6–12 μm, tetrasterigmate, clavate, some with median constriction | 24–33 × 7–11(12) μm, tetrasterigmate, clavate | 18–27 × 6–10 μm, tetrasterigmate, clavate, some with median constriction | 18–30 × 5.5–8 μm, tetrasterigmate, constricted in the middle |
| Pleurocystidia | 50–100 × 12–24(–30) μm, fusiform to narrowly utriform, 2–4 apical hooks, 3 μm thick-walled | 49–75(78) × (13)15–26 μm, fusiform or narrowly fusiform to narrowly utriform, with 2–4 apical hooks (commonly entire), 2–3 μm thick-wall | 60–97 × 12–22(–29) μm, fusiform to utriform, 2–4 apical hooks, sometimes lateral hooks, 3 μm thick-walled | 70–90 × 10–25 μm, fusiform or utriform, 2–3 apical hooks, 2.7 μm thick-walled |
| Cheilocystidia | 30–68 × 12–22 μm, clavate to spheropedunculate, hyaline, thin-walled | 38–60 × (11)12–15 μm, crowded, grouped to clustered, clavate or narrowly clavate to capitate, with obtuse at apices | (27–)34–55 (65) × 12–20(–29) μm, clavate, hyaline, thin-walled | 30–55 × 13–23 μm, clavate to utriform, hyaline, thin-walled |
| Intermediate cystidia | irregularly shaped, thin-walled | fusiform, poke-shaped, with pointed or flatter apices, smaller than the pleurocystidia, thick-walled | irregularly shaped, thin-walled | inflated fusiform, thin-walled |
| Lamellar edge | Lamellar edge sterile | Lamellar edge sterile | Lamellar edge sterile | Lamellar edges sterile |
| Pileipellis a cutis | 80–146 × 8–15 μm, cylindrical, strongly tapering towards apex, brown intracellular pigment, thin-smooth walled | 80–146 × 6–18 μm, individual elements cylindrical, tapering towards apex, filled with brown intracellular pigment, thin-smooth walled | 90–150 × 7–17 μm, cylindrical, strongly tapering towards apex, brown intracellular pigment, thin-smooth walled | 70–110 × 12–20 μm, cylindrical or slightly inflated, tapering towards apex, hyaline or pale yellow-brown intracellular pigment, thin-walled |
| Stipitipellis a cutis | 5–25 μm wide, cylindrical, hyaline or brown pigment, thin-smooth walled | 6–23 μm wide, cylindrical, hyaline or brown intracellular pigment, thin-smooth walled | 5–20 μm wide, cylindrical, hyaline or brown pigment, thin-smooth walled | 7–20 μm wide, cylindrical, hyaline or with yellow-brown intracellular pigment, thin-smooth walled |
| Clamp-connections | common and readily seen on pileipellis hyphae | absent in all tissues | common and readily seen on pileipellis hyphae | present on pileipellis hyphae |
| Ecolog | Solitary or gregarious | Solitary | Solitary or subgregarious | Subgregarious |
| Habit | growing on decayed wood of hardwoods or on the humus layer under hardwoods or conifers | growing on decayed wood of conifers in summer | growing on well-decayed wood of hardwoods, in hardwood-dominated or mixed forests | growing on well-decayed wood of conifers |
| Distribution | Russian, America, Turkey | China | Japan, Russian | Russian |
The type specimen of P. brunneidiscus (Murrill, 1917) collected in Connecticut has a high similarity growth environment to our specimens, both in a temperate climate and autumn. We provide a detailed description of the morphological characteristics of P. brunneidiscus, which enriches Murrill’s (Murrill, 1917) report, such as the color variation of the pileus and the size range of the spores. At the same time, there are some differences. For example, the pileus of margin of our specimens has slightly sticky umbilical protrusions. They sometimes have translucent stripes at the pileus margin, while clamp connections are absent.
Pluteus hongoi Singer, Fieldiana, Bot. 21: 95 (1989).
Syn.: P. major Singer, Fieldiana, Bot. 21: 96 (1989);
Syn.: P. albineus Bonnard, Mycol. helv. 11(2): 131 (2001);
Syn.: P. nothopellitus Justo & M.L. Castro, Mycotaxon 102: 222 (2007).
Figure 7: Microscopic features of Pluteus hongoi.
(A) Cheilocystidia. (B) Pileipellis. (C) Basidiospores. (D) Basidia. (E) Pleurocystidia. Scalebars: (C) 5 μm; (D) 10 μm; (A, B, E) 20 μm.Description
Basidiomata medium-sized. Pileus about 36.6 mm broad, flattened hemispherical, surface smooth and shiny, dark brown at the disc (7.5YR 4/2-7.5YR 6/2), becoming lighter toward the margin, margin transversely folded. Lamellae close, free, dense, folded, gray-white to earthy yellow (7.5YR 9/6-7.5YR 9/4), and slightly ventricose. Stipe 40 mm × 5.2 mm, central, subterete, fibrous, slightly expanded downward, white (7.5YR 9/2), smooth, with white longitudinally ciliate at the base. Spore print unknown.
Basidiospores [60, 1, 1] (7.0)8.0–9.5(10.0) × 5.5–6.5 μm, avl = 9.0 μm, avw = 6.0 μm, Q = 1.33–1.60(1.66), Qm = 1.50 ± 0.10, oval, pink, thin-walled, smooth, non-dextrinoid. Basidia 26–38 × 8–11 μm, clavate, usually 4-sterigmate, thin-walled, and hyaline in KOH. Pleurocystidia 55–83 × 14–23 μm, crowded, covering the whole side of the lamellae, flask-shaped to fusiform with 2–5 apical hooks (usually bifid), thick-walled, neck thickness up to 2–3 μm, hyaline in KOH. Cheilocystidia 29–57 × 12–20 μm, abundant, clavate or broad clavate to capitate, obtuse apices, hyaline, thick-walled. Pileipellis a cutis, with terminal elements 69–153 × 5–17 μm; individual elements cylindrical, some strongly tapering towards apex, mostly filled with brown intracellular pigment, smooth, and thin-walled. Stipitipellis a cutis, hyphae 5–19 μm wide, cylindrical, hyaline or brown intracellular pigment, smooth, and thin-walled. Clamp connections absent in all tissues.
Ecology. Solitary or scattered on rotten wood in mixed forests in summer and autumn.
Distribution. Japan, Spain, Russia, USA, Germany, Czech Republic, Slovakia, Republic of Korea, Turkey (Justo et al., 2014; Ševčíková, 2016; Kaygusuz et al., 2021), and China (Heilongjiang, Sichuan, Xinjiang Uygur Autonomous Region) (Xu, 2016).
Specimen examined. CHINA. Xinjiang Uygur Autonomous Region, Aletai Region, Aletai City, Wolong Bay in Kanas, 48°65′77.01″N, 87°03′82.11″E, ASL1333 m, 16 September 2021, Z.X. Qi, D.M. Wu, N. Gao & B.K. Cui, HMJAU60205 (OM302007).
Note. Singer (1989) first described P. hongoi and P. major Singer. Later, P. hongoi is recognized by Justo et al. (2014), because the original description of this taxon is more complete than P. major. Compared with our specimen, the type specimens (Singer, 1989) have smaller basidiospores, and the apical hook’s structure was often dehiscent.
Justo et al. (2014) studied the species belong to section Pluteus in the Holarctic region. They combined the molecular data based on type specimens of P. major, P. albineus Bonnard (Bonnard, 2001), and P. nothopellitus Justo & Castro (2007b) indicate that all these species represent different morphological variants of P. hongoi.
Pluteus hongoi was easily confused with P. cervinus. The pileus of P. cervinus (Murrill, 1917; Justo et al., 2014) was very variable in colors (brown, gray-brown, orange-brown, white), aspect of the pileus (with or without conspicuous squamules and radial fibrils) the stipe often had longitudinal and brown, or gray-brown fibers or scales, and the apical hooks structure (commonly entire) of the pleurocystidia. However, these features are variable and cannot be easily expressed on a basidiocarp (Justo et al., 2014). But they can be distinguished by sequences analyses.
Key to the reported species of Pluteus in Xinjiang Uyghur Autonomous Region
1. Metuloid cystidia...............................................................................................................................................2
- Non-metuloid cystidia.........................................................................................................................................5
2. Pleurocystidia apical hooks commonly entire...................................................................................................3
- Pleurocystidia apical hooks usually bifid..............................................................................................P. hongoi
3. With intermediate cystidia.................................................................................................................................4
- Without intermediate cystidia...............................................................................................................P. pellitus
4. Stipe has longitudinal brown stripes, basidiospores (7.0)7.5–8.0(9.0) × 5.0–6.0, pleurocystidia 2–4 apical hooks..........................P. brunneidiscus
- Stipe has continuous longitudinal brown fibrillose, basidiospores 5.5-8(-9) × 4.5-7, pleurocystidia 3–5 apical hooks....................................P. cervinus
5. Pileipellis a trichoderm.....................................................................................................................................6
- Pileipellis a non-trichoderm................................................................................................................................7
6. Pileus black-brown.........................................................................................................................P. umbrosus
- Pileus golden to dull or brownish yellow............................................................................................P. leoninus
7. Pleurocystidia and cheilocystidia with tapered apices....................................................................P. thomsonii
- Pleurocystidia and cheilocystidia without tapered apices................................................................P. aletaiensis
Discussion
In this study, one new species, Pluteus aletaiensis, a new record species from China, P. brunneidiscus, and a new record species from Xinjiang Uyghur Autonomous Region, China, P. hongoi, were discovered in Xinjiang Uyghur Autonomous Region based on morphological studies and phylogenetic analyses.
P. brunneidiscus without clamp connections is very interesting. Clamp connections are an essential discriminating character in the brunneidiscus clade, and in the articles of Justo & Castro (2007a), Justo et al. (2014), and Kaygusuz et al. (2021), P. brunneidiscus are present clamp connections; however, our specimen does not have this feature in all tissues, which is unusual and could be caused by the environmental specificity here, and it was growing on a mainly decayed Picea schrenkiana Fischet Mey. stump.
Most species of the genus Pluteus are saprophytic on trees (conifers or angiosperms). Some of them are also indirectly associated with trees, e.g., P. cervinus, P. hongoi, P. elaphinus Justo, P. petasatus (Fr.) Gillet, P. pellitus (Pers.) P. Kumm., P. salicinus (Pers.) P. Kumm., P. fulvibadius, P. amphicystis Singer, and P. americanus (P. Banerjee & Sundb.) Justo, E.F. Malysheva & Minnis, which are always found to grow on angiosperm wood or in the humus layer without apparent connection to wood (mostly P. hongoi and P. petasatus) or piles of woodchips (especially P. petasatus and P. pellitus) (Justo et al., 2014). However, there is also a small percentage of species that are only found on the ground, without association with trees. e.g., P. ephebeus (Fr.) Gillet, P. fenghuangensis Z.S. Bi, P. nankungensis Z.S. Bi & T.H. Li, and P. aletaiensis. In summary, there are some adaptation mechanisms between these species and trees or ecology, e.g., growing on decaying material of specific tree species or decaying material in all tree species, growing on decaying material of live trees/fallen wood/dead trees/stumps, etc., indirectly having some association with trees, growing on the ground/humus, not associated with trees. In the future, subsequent studies of Pluteus will require additional data to further support it.