Metagenomics analysis of the effects of Agaricus bisporus mycelia on microbial diversity and CAZymes in compost
- Published
- Accepted
- Received
- Academic Editor
- Ravinder Kumar
- Subject Areas
- Agricultural Science, Microbiology, Molecular Biology, Mycology
- Keywords
- Agaricus bisporus, Metagenome, Community succession, CAZymes, Lignocellulose, Laccase, Xylanase
- Copyright
- © 2022 Chang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Metagenomics analysis of the effects of Agaricus bisporus mycelia on microbial diversity and CAZymes in compost. PeerJ 10:e14426 https://doi.org/10.7717/peerj.14426
Abstract
Agaricus bisporus growth alters the lignocellulosic composition and structure of compost. However, it is difficult to differentiate the enzyme activities of A. bisporus mycelia from the wider microbial community owing to the complication of completely speareting the mycelia from compost cultures. Macrogenomics analysis was employed in this study to examine the fermentation substrate of A. bisporus before and after mycelial growth, and the molecular mechanism of substrate utilization by A. bisporus mycelia was elucidated from the perspective of microbial communities and CAZymes in the substrate. The results showed that the relative abundance of A. bisporus mycelia increased by 77.57-fold after mycelial colonization, the laccase content was significantly increased and the lignin content was significantly decreased. Analysis of the CAZymes showed that AA10 family was extremely differentiated. Laccase-producing strains associated with AA10 family were mostly bacteria belonging to Thermobifida and Thermostaphylospora, suggesting that these bacteria may play a synergistic role in lignin decomposition along with A. bisporus mycelia. These findings provide preliminary evidence for the molecular mechanism of compost utilization by A. bisporus mycelia and offer a reference for the development and utilization of strains related to lignocellulose degradation.
Introduction
Agricultural biomass wastes comprise organic substances generated by humans during agricultural activities (Malool, Keshavarz Moraveji & Shayegan, 2021). As these wastes are produced in abundant quantities and pose disposal problems, there has been an increasing interest to develop efficient and safe strategies to utilize agricultural biomass waste (Sherwood, 2020; Grimm & Wösten, 2018). At present, compost is still a primary mode of organic matter degradation (Wang et al., 2021a and Wang et al., 2021b).
Industrial-scale production of Agaricus bisporus, an edible mushroom with a long history of cultivation (Baars et al., 2020), has solved part of the problem of agricultural waste reuse to a certain extent (De Andrade et al., 2008). In the process of large-scale production of A. bisporus, agricultural wastes, such as wheat straw and chicken manure, are mainly used as raw materials for fermentation (Roncero-Ramos & Delgado-Andrade, 2017), which is both environmentally-friendly and economical, addressing the issue of reusing of agricultural waste to a certain extent (Colmenares-Cruz, Sánchez & Valle-Mora, 2017). In recent years, significant improvements in the A. bisporus cultivation process have been achieved, and the application of tunnel inoculation has altered the cultivation pattern and increased the mycelial growth rate. It has been reported that the localized tunnel-growth model achieved a 13.6% increase in A. bisporus growth rate, when compared with the cultivation house growth model (Wang et al., 2021a; Wang et al., 2021b). However, only a few studies have performed comparative investigations of inoculated and uninoculated mushroom compost. Some studies have suggested that A. bisporus mycelial growth produces a range of extracellular enzymes that are involved in the degradation of the lignocellulosic fraction in compost. Lignin is mainly degraded during A. bisporus mycelial growth stage (PIII), with an increase in guaiacyl lignin content (G-type lignin) (Wood & Leatham, 1983), and the lignin-degradation products have been speculated to be the substrate for subsequent growth of A. bisporus (Jurak et al., 2015; Jurak, Kabel & Gruppen, 2014). The decrease and changes in lignin during the mycelial growth stage can improve the digestibility of carbohydrates in the later growth phases. In a previous study, A. bisporus appeared to be the dominant fungal species based on visual observation of cropping beds. However, phospholipid fatty acid analysis (PLFA) conducted on mushroom compost revealed that A. bisporus mycelia accounted for 6.8% w/w of the mushroom compost after complete colonization, with only less than half of the mycelia being active (McGee, 2017).
Many studies have focused on the crucial role of bacteria and fungi in the degradation of organic compoundsand their diverse modes of action on organic matter decomposition. While bacterial growth becomes restricted owing to their enhanced propagation on the surface of organic matter that acts as the main source of nutrients, the fungal hyphae have a strong penetrating ability. About concerning Agaricus growth, both mycelia and fruiting body production are not only dependent on the mushroom itself, but also bacteria and other fungi in the substrate, and the microbial community dynamics can completely change at the end of the composting process (Song et al., 2021). It has been noted that the final secondary fermentation (PII) compost mainly comprised lignocellulosic components from wheat straw together with microbial biomass (Martínez et al., 2008). Pasteurization of the compost material before inoculation has been found to result in the predominance of fungal community in the substrate, with A. bisporus becoming the major fungal strain and its mycelia subsequently colonizing the substrate by degrading the organic material to release nutrients (McGee, 2018). However, little is known about the composition and activity of the wider fungal community in the compost substrate besides A. bisporus throughout the mushroom cultivation process. Therefore, the present study aimed to reveal the utilization of compost substrate before and after A. bisporus mycelial growth and compare the differences in the microbial communities and enzyme families in the compost. Furthermore, the effects of A. bisporus mycelial growth on other microorganisms during large-scale cultivation of A. bisporus were determined to identify novel microorganisms with potential roles in lignin degradation.
Materials & Methods
Sample collection and DNA extraction
Commercial strain A15 of A. bisporus from Sylvan (USA) was used in this study and stored in the Engineering Research Center of Chinese Ministry of Education for Edible and Medicinal Fungi (ERCCMEEMF) at Jilin Agricultural University (Changchun, China). The compost fermentation and mycelial culture experiments were performed at Zhejiang Longchen Modern Agricultural Science and Technology Co. Ltd, Jiaxing City, Zhejiang Province, China. The compost comprised wheat straw (90 t), chicken manure (83 t), peanut meal (3 t), and gypsum (9 t). Peanut meal was added as an auxiliary nitrogen source because the components of wheat straw and chicken manure in China were different from those in Europe and America (Jun et al., 2021). Before commencing compost fermentation, the initial C/N ratio of the compost was adjusted to 25:1. The straws were completely dampened and piled up for 1–3 days, and then the other materials were mixed and piled again for 2–3 days. The pre-compost was placed into the tunnel for primary fermentation (PI) (Straatsma et al., 2000; Mouthier et al., 2017). The pile was turned three times on days 2, 4, and 7, respectively. The treatment parameters were adjusted based on compost temperature to allow the material temperature to reach 70 °C–80 °C and remain constant for 6 days (PI). Immediately after that, secondary fermentation (Compost-PII) was conducted for 6–7 days by pasteurization (Vieira & Pecchia, 2018). After secondary fermentation, inoculation was performed when the temperature decreased to 24 °C and NH3 level was ≤10 mg/L (Sharma, Lyons & Chambers, 2005). The temperature, humidity, air pressure, air volume, and other environmental factors were adjusted using Dutch Christiaens Group equipment for the intelligent control system. Under optimal environmental conditions, A. bisporus mycelia could grow all over the compost after 18 days (Mycelium-PIII) (Iiyama, Stone & Macauley, 1994).
Subsequently, samples were collected from the uninoculated compost and mycelia-filled compost, respectively. Before sample collection, the composts in the top, middle, and bottom layers of the reactor were fully mixed (Meng et al., 2021). All the samples were divided into two parts: one part was stored at 4 °C for physicochemical analysis and the other was frozen at −80 °C for DNA extraction. The genomic DNA was extracted from the samples using an Omega EZNA soil DNA kit, and the integrity, purity, and concentration of the extracted genomic DNA were examined by 1% agarose gel electrophoresis (100 V, 1.5 h), NanoDrop 2000, and Qubit 3.0, respectively. The extracted DNA was stored in an ultra-low-temperature freezer at −20 °C and transported to OE Biomedical Technology (Shanghai, China) for sequencing.
Analysis of compost physicochemical properties
The compost samples were dried in an oven at 105 °C for 5 h to assess the moisture content. The dried samples were crushed and placed in the control box of a resistance furnace at 600 °C for 2 h to determine the ash content. The total nitrogen (Total-N) and carbon (Total-C) content in the samples were determined using the Kjeldahl method and K2Cr2O3 oxidation. Determination of the pH values with an electronic pH meter (Mettler-Toledo Instruments Co., Ltd., Shanghai, China) using 10%(w/v) sample suspensions were used. Laccase and xylanase activities in the samples were evaluated using a Solarbio kit, and lignin, cellulose, and hemicellulose components were determined according to Van Soes method.
Metagenome sequencing, assembly, and annotation
Metagenome sequencing was accomplished using the Illumina HiSeq platform with a 500 bp sequencing library. The raw data (raw reads) quality was pre-processed using Trimmomatic (Bolger, Lohse & Usadel, 2014) software, and optimized sequences were spliced and assembled using MEGAHIT (Li et al., 2015; Li et al., 2016) software based on De-Bruijn graph principle, and contigs with length <500 bp were filtered out for subsequent analysis. The open reading frame (ORF) of the spliced contigs was predicted with Prodigal software (Hyatt et al., 2010). CD-HIT software was adopted to remove redundant and non-redundant initial unigenes. The clustering parameters included 95% identity and 90% coverage. The clean reads of each sample were aligned to the non-redundant genes set (95% identity) using bowtie 2 software to calculate the gene abundance in the corresponding samples. The representative sequences in the non-redundant unigenes set were annotated to the obtained species information according to the best alignment attained by BLASTP (E value<1e−5) to National Center for Bio-technology Information (NCBI) Non-Redundant Database (Nr). Then, the sum of gene abundances for the corresponding species was used to calculate species abundance.
Identification of carbohydrate-active enzymes
To evaluate the carbon utilization potential of microbial communities during A. bisporus mycelial growth, the non-redundant genes were compared with the carbohydrate-active enzymes database (CAZy) using DIAMOND software (e<1e−5) (Buchfink, Xie & Huson, 2015). First, all proteins with the highest sequence similarity were screened and subjected to CAZy to search against sequence libraries with the families of glycoside hydrolases (GHs), auxiliary activities (AAs), carbohydrate-binding modules (CBMs), glycosyltransferases (GTs), polysaccharide lyases (PLs), and carbohydrate esterases (CEs). Then, the differences in the CAZyme family between the two samples (uninoculated compost and mycelia-filled compost) were compared and analyzed (Donhauser et al., 2021).
Data and statistical analyses
Raw data were entered and stored in Excel. The differences among the samples were examined by independent samples t-tests with statistical significance at p < 0.05 and p < 0.01. The data are presented as mean ± standard deviation (SD). GraphPad Prism 8.0 software and Origin 2021 were applied for statistical analysis and plotting, and a cloud platform (http://www.cloudtutu.com/) was employed for plotting.
Results
Physicochemical properties of Compost-PII and Mycelium-PIII
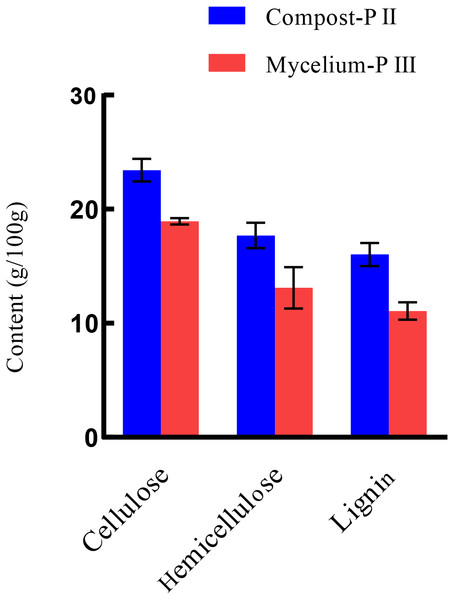
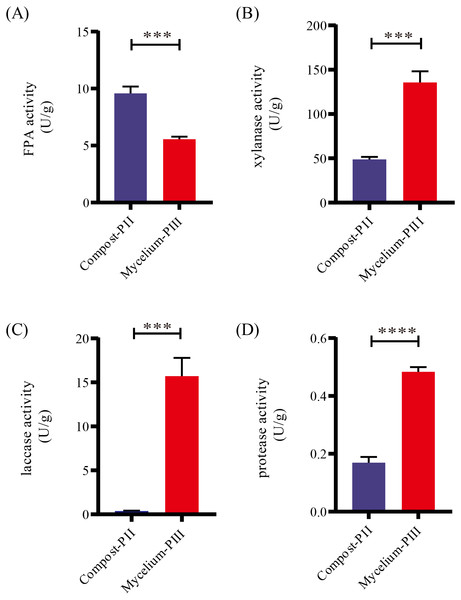
After completion of PII, the material temperature was reduced using fans. Subsequently, A. bisporus was inoculated (4‰ (w/w)) and the compost substrate was filled with mycelial growth after 18 days of incubation at 22 °C–24 °C. During this period, the water content in the compost decreased from 66.48% to 61.77% with the increase in mycelial growth, whereas the ash content increased by 1.71% (Table 1). It must be noted that the water content can affect microbial activities, which in turn can influence enzyme activities. During A. bisporus mycelial growth, carbon consumption predominantly increased, whereas nitrogen utilization was relatively less maintained at 2.12–2.13% and no significant difference (Table 1). After mycelial growth, the pH of the compost decreased from 7.80 to 6.27. Analysis of the cellulose and lignin contents in the compost by Van Soes method revealed that the cellulose content and lignin content significantly decreased (Fig. 1). Furthermore, evaluation of the activities of several known carbon source degradation-related enzyme families indicated a moderate increase in xylanase and laccase activities after A. bisporus mycelial growth, three-fold and 15-fold increase respectively (Figs. 2B & 2C). The solubility of lignin in aqueous solutions was low, and the decrease in the pH of the culture material may have a significant effect on lignin solubility. In addition, the increase in protease (Fig. 2D) also promotes better development of the mycelium (Wang et al., 2021a and Wang et al., 2021b).
| Phase | Total carbohydrates (w/w%) | Total nitrogen (w/w%) | Moisture(%) | Ash(%) | pH | |
|---|---|---|---|---|---|---|
| Compost-II | T1 | 28.48 ± 0.20 | 2.12 ± 0.03 | 66.46 ± 0.60 | 33.6 ± 1.69 | 7.85 ± 0.09 |
| T2 | 28.63 ± 0.18 | 2.14 ± 0.07 | 66.11 ± 0.25 | 33.54 ± 1.06 | 7.65 ± 0.25 | |
| T3 | 28.58 ± 0.06 | 2.18 ± 0.08 | 66.95 ± 1.74 | 33.96 ± 0.79 | 7.90 ± 0.20 | |
| Mycelium-III | T1 | 23.59 ± 0.22 | 2.05 ± 0.02 | 59.50 ± 4.14 | 36.00 ± 1.22 | 6.30 ± 0.01 |
| T2 | 23.74 ± 0.15 | 2.06 ± 0.02 | 61.80 ± 0.86 | 36.20 ± 1.36 | 6.24 ± 0.02 | |
| T3 | 23.44 ± 0.28 | 2.13 ± 0.06 | 63.00 ± 1.47 | 34.10 ± 2.61 | 6.30 ± 0.04 | |
Notes:
Notes. T1,T2,T3: different trials; Results represent mean ± standard deviation (n = 3).
Figure 1: The lignocellulose contents of Compost-PII and Mycelium-PIII.
Lignocellulose: cellulose, hemicellulose, lignin. Compost-PII: end of the compost; Mycelium-PIII: A. bisporus mycelia could grow all over the compost after 18 days. (* p < 0.05, ** p < 0.01) (n = 3).Figure 2: Enzyme activities in various stages.
(A) FPA activity; (B) xylanase activity; (C) laccase activity; (D) protease activity. Note: * P < 0.05. ** P < 0.01. *** P < 0.001. **** P < 0.0001.Diversity of microbial communities in Compost-PII and Mycelium-PIII
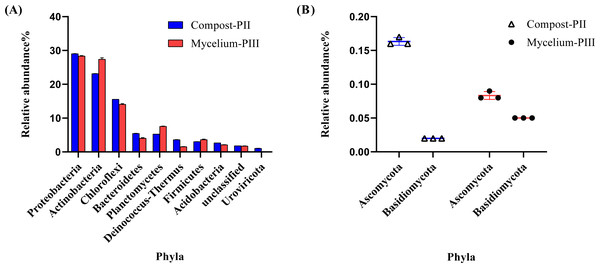
The effective data volume of each sample in this experiment was 11.23–17.22 G. The N50 statistics of Contigs were distributed between 1631–2349 bp, and the number of OFR in the set of non-redundant genes was 1029162 after redundancy. The annotation rates were 89.44%, 74.62%, 42.21% and 2.57% for the non-redundant genes compared with NR, eggNOG, KEGG and CAZy databases respectively. Metagenomics analysis indicated the dominance of bacterial community in Compost-PII and Mycelium-PIII samples (93.17% and 94.27%, respectively), followed by fungi (0.25% and 0.29%, respectively), whereas the archaeal abundance remained almost unchanged. However, the abundance of viruses declined with A. bisporus mycelial growth (1.18% in Compost-PII and 0.13% in Mycelium-PIII). A total of 181 phyla, 157 classes, 805 families, 3460 genera, and 22,567 species were detected in the samples. The six most prominent bacterial phyla were Proteobacteria, Actinobacteria, Chloroflexi, Planctomycetes, Bacteroidetes, and Firmicutes (Zhang et al., 2014), and the abundances of Actinobacteria and Planctomycetes significantly increased after A. bisporus mycelial growth (Fig. 3A). Intriguingly, numerous lignocellulose-decomposing bacteria have been reported to belong to Proteobacteria, Firmicutes, Actinobacteria, and Bacteroidetes (Lewin, Wentzel & Valla, 2013; Pankratov et al., 2011). Proteobacteria and Bacteroidetes are known to play a major role in organic matter degradation and C cycling (Wang, Mao & Li, 2018), and Bacteroidetes can break down lignocellulose into short-chain fatty acids (Dodd, Mackie & Cann, 2011). It is noteworthy that although the relative abundance of the Basidiomycota phylum was low, it showed a great increase (Fig. 3B).
Figure 3: Microbial community analysis of Compost-PII and Mycelium-PIII (phylum level).
(A) Relative abundance of microbial communities (Top 10 phyla level). (B) Relative abundance of Ascomycota and Basidiomycota (phylum level).Analysis of bacterial and fungal communities
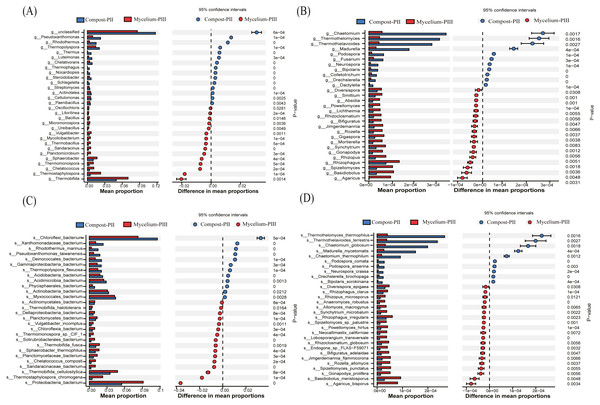
In the present study, Thermobifida, Thermostaphylospora, Sphaerobacter, Thermopolyspora, Pseudoxanthomonas, and Rhodothermus were the predominant bacterial genera in the Compost-PII and Mycelium-PIII samples (Cao et al., 2019; Durrant, Wood & Cain, 1991). When compared with the Compost-PII samples, the relative abundances of Thermobifida, Thermostaphylospora, Sphaerobacter, Thermomonospora, and Chelatococcus were significantly increased in Mycelium-PIII samples; in contrast, the relative abundances of Thermopolyspora, Rhodothermus, and Pseudoxanthomonas presented the opposite trend (Fig. 4A). Analyses of the microbial community composition confirmed significant shifts in the microbial community structure between the two groups, and many of the enriched genera also co-varied with function. Moreover, the variability of these microbial communities may be correlated with nutrients, compost temperature, moisture content, and pH.
Figure 4: Significant differences between fungal and bacterial communities for compost-PII and mycelium-PIII on the genus level.
(A) Bacterial community; (B) fungal community and species level; (C) bacterial community; (D) fungal community.At the species level, the relative abundance of A. bisporus presented the highest increase among fungi, exhibiting a 77.57-fold increase after complete mycelial growth (Fig. 4D). In addition to A. bisporus, the activities of other microorganisms, such as the bacteria (Fig. 4C) Thermostaphylospora_chromogena, Thermomonospora_sp._CIF_1, Sandaracinaceae_bacterium, and Chelatococcus_composti, and fungi (Fig. 4D) Spizellomyces_punctatus, Rozella_allomycis, and Basidiobolus_meristosporus, were also enhanced during A. bisporus mycelial growth.
Analysis of CAZymes
The carbon-utilization potential of the microbial communities in the compost was assessed to evaluate the effects of altered substrate quantity and quality resulting from the shift in microbial activity during A. bisporus mycelial growth. Among the genes annotated with CAZy, in total, 431 different CAZyme families (229 GHs, 81 GTs, 48 PLs, 17 AAs, 16 CEs, and 40 CBMs) were detected in the samples. The most abundant enzyme classes at all temperatures were GHs and GTs, whereas those with the lowest abundance were PLs and AAs. At the family level, GTs were especially abundant in all the samples, with GT2 (cellulose/chitin synthase and other functions), GT4 (sucrose synthase and other functions), and GT83 (galacturonosyl transferase and other functions) being the most abundant (Paixão et al., 2021; Leadbeater et al., 2021).
Cellulose, hemicellulose, and lignin are major constituents of lignocellulose-containing raw materials (Stech et al., 2014). In the present study, cellulose- and hemicellulose-degrading enzymes exhibited the highest activities in Compost-PII and Mycelium-PIII samples, with GH5, GH8, and GH9 families being the predominant cellulose-degrading enzymes and GH2, GH10, GH11, GH26, and GH53 families being the major hemicellulose-degrading enzymes (Table 2). The GH2 family includes multiple enzymes, and it has been demonstrated that α-1,3-L-arabinofuranosidase activity on substituted xylan does not improve compost degradation by A. bisporus. In nature, it is generally attributed to the metabolism of basidiomycetes white-rot fungi, since they degrade lignin more rapidly and extensively than other microorganisms (Woiciechowski et al., 2013). Although not a wood-rotting fungi, the Agaricus bisporus still plays a key role in the degradation of lignin as a grass-rotting fungi. Although AA7 family genes have been reported to play a role in lignin degradation (Andlar et al., 2018) and the content of these genes was relatively high in the lignin-degradation-related enzymes family in the present study, this enzymes family was not significantly different. There is a large content of the AA3 and AA6 enzyme families associated with lignin breakdown (Table 2).
| CAZy family | Annotation | Genes_Count | Total putative CAZy genes, ≥50% Covered Fraction | Total putative CAZy genes, 50–70% Covered Fraction | Total putative CAZy genes, ≥90% Covered Fraction |
|---|---|---|---|---|---|
| Cellulose degrading | |||||
| GH5(-) | Endo-beta-1,4-glucanase ;cellulase | 73 | 50 | 19 | 0 |
| GH6(-) | Endoglucanase; cellobiohydrolase | 31 | 26 | 4 | 19 |
| GH8(↓) | chitosanase ;cellulase;licheninase | 60 | 49 | 13 | 31 |
| GH9(-) | Endoglucanase ; endo-beta-1,3(4)-glucanase | 152 | 94 | 21 | 47 |
| GH12(-) | Endoglucanase ; xyloglucan hydrolase | 38 | 36 | 0 | 33 |
| GH140(-) | Apiosidase | 41 | 24 | 8 | 9 |
| GH44(-) | Endoglucanase; xyloglucanase | 24 | 15 | 5 | 9 |
| GH116(-) | Beta-glucosidase ;beta-xylosidase | 24 | 14 | 4 | 8 |
| GH3(-) | Beta-glucosidase ; xylan 1,4-beta-xylosidase | 471 | 382 | 87 | 222 |
| GH48(-) | Endo-beta-1,4-glucanase ;chitinase | 13 | 4 | 1 | 2 |
| Hemicellulose degrading | |||||
| GH10(-) | Endo-1,4-beta-xylanase ;endo-1,3-beta-xylanase | 223 | 168 | 56 | 78 |
| GH11(-) | Endo-beta-1,4-xylanase ;endo-beta-1,3-xylanase | 35 | 31 | 3 | 27 |
| GH1(-) | Beta-glucosidase; beta-galactosidase | 217 | 136 | 34 | 72 |
| GH42(↑) | Beta-galactosidase; alpha-L-arabinopyranosidase | 60 | 31 | 13 | 10 |
| GH2(-) | Beta-galactosidase ; beta-mannosidase; beta-glucuronidase; alpha-L-arabinofuranosidase | 151 | 81 | 66 | 7 |
| GH26(-) | Beta-mannanase; beta-1,3-xylanase | 67 | 32 | 0 | 14 |
| GH27(↓) | Alpha-galactosidase; alpha-N-acetylgalactosaminidase | 10 | 6 | 6 | 0 |
| GH3(-) | Beta-glucosidase; xylan 1,4-beta-xylosidase; beta-glucosylceramidase | 471 | 382 | 87 | 222 |
| GH31(-) | Alpha-glucosidase; alpha-galactosidase; alpha-mannosidase; alpha-1,3-glucosidase | 164 | 102 | 36 | 54 |
| GH38(-) | Alpha-mannosidase | 64 | 55 | 15 | 32 |
| GH39(-) | Alpha-L-iduronidase; beta-xylosidase | 196 | 108 | 85 | 5 |
| GH4(-) | Maltose-6-phosphate glucosidase; alpha-glucosidase | 79 | 69 | 11 | 52 |
| GH43(-) | Beta-xylosidase; alpha-L-arabinofuranosidase; xylanase | 5 | 2 | 0 | 1 |
| GH5(-) | Endo-beta-1,4-xylanase; beta-glucosidase; beta-mannosidase | 73 | 50 | 19 | 0 |
| GH53(-) | Endo-beta-1,4-galactanase | 19 | 15 | 4 | 5 |
| GH92(-) | Mannosyl-oligosaccharide alpha-1,2-mannosidase; mannosyl-oligosaccharide alpha-1,3-mannosidase | 62 | 30 | 14 | 13 |
| GH30(-) | Endo-beta-1,4-xylanase; beta-glucosidase; beta-glucuronidase | 19 | 11 | 3 | 7 |
| CE1(-) | Acetyl xylan esterase; cinnamoyl esterase | 730 | 663 | 244 | 163 |
| CE4(-) | Acetyl xylan esterase; chitin deacetylase | 623 | 589 | 87 | 251 |
| CE7(-) | Acetyl xylan esterase; cephalosporin-C deacetylase | 123 | 74 | 22 | 33 |
| CE15(-) | 4-O-methyl-glucuronoyl methylesterase | 111 | 101 | 36 | 51 |
| CE6(-) | Acetyl xylan esterase | 23 | 22 | 2 | 20 |
| GH78(-) | Alpha-L-rhamnosidase | 165 | 60 | 28 | 24 |
| GH28(-) | Polygalacturonase; alpha-L-rhamnosidase | 39 | 22 | 11 | 7 |
| GH35(-) | Beta-galactosidase; beta-1,3-galactosidase | 34 | 25 | 12 | 11 |
| PL1(↑) | Pectate lyase; pectin lyase | 9 | 9 | 0 | 2 |
| PL9(-) | Pectate lyase | 76 | 22 | 11 | 1 |
| CE8(↓) | Pectin methylesterase | 16 | 8 | 5 | 3 |
| CE12(-) | Pectin acetylesterase; acetyl xylan esterase | 20 | 20 | 1 | 16 |
| GH18(-) | Chitinase; lysozyme | 129 | 77 | 30 | 21 |
| GH19(-) | Chitinase; lysozyme | 22 | 9 | 6 | 2 |
| GH3(-) | Beta-glucosidase; xylan 1,4-beta-xylosidase; beta-glucosylceramidase | 471 | 382 | 87 | 222 |
| CE4(-) | Acetyl xylan esterase; chitin deacetylase | 623 | 589 | 87 | 251 |
| GH13(-) | Alpha-amylase; pullulanase | 77 | 59 | 15 | 28 |
| GH23(-) | Lysozyme type G; chitinase | 568 | 539 | 93 | 22 |
| GH51(-) | Endoglucanase; endo-beta-1,4-xylanase; beta-xylosidase | 118 | 62 | 20 | 0 |
| GH67(-) | Alpha-glucuronidase; xylan alpha-1,2-glucuronidase | 58 | 30 | 8 | 13 |
| GH127(-) | Beta-L-arabinofuranosidase ; 3-C-carboxy-5-deoxy-L-xylose | 56 | 31 | 9 | 18 |
| GH32(-) | Endo-inulinase; | 43 | 38 | 10 | 20 |
| GH97(↓) | Glucoamylase; alpha-glucosidase; alpha-galactosidase | 41 | 21 | 9 | 11 |
| GH16(-) | Licheninase | 106 | 94 | 24 | 42 |
| GH29(-) | Alpha-L-fucosidase; alpha-1,3/1,4-L-fucosidase | 65 | 48 | 14 | 14 |
| GH95(-) | Alpha-L-fucosidase ; alpha-1,2-L-fucosidase; alpha-L-galactosidase | 53 | 30 | 13 | 13 |
| GH94(-) | Cellobiose phosphorylase ;laminaribiose phosphorylase | 54 | 29 | 15 | 2 |
| GT35(-) | Glycogen or starch phosphorylase | 162 | 92 | 69 | 11 |
| Lignin degrading | |||||
| AA1(↓) | Laccase/ferroxidase | 1 | 0 | 0 | 0 |
| AA2(-) | Manganese peroxidase ; versatile peroxidase ;lignin peroxidase | 39 | 12 | 1 | 7 |
| AA3(-) | Ellobiose dehydrogenase;glucose 1-oxidase | 201 | 122 | 119 | 0 |
| AA5(-) | Alactose oxidase;glyoxal oxidase ; alcohol oxidase | 8 | 0 | 0 | 0 |
| AA6(-) | 1,4-benzoquinone reductase | 98 | 86 | 10 | 61 |
| AA7(-) | Glucooligosaccharide oxidase; chitooligosaccharide oxidase | 405 | 140 | 68 | 49 |
| AA10(↑) | Lytic chitin monooxygenase | 22 | 21 | 0 | 20 |
Notes:
“ ↑” and “ ↓” indicate the up-regulation and down-regulation of the relative abundance for differential CAZy, “-” indicate that there is no difference between the two comparison groups.
Variations in enzyme families between Compost-PII and Mycelium-PIII
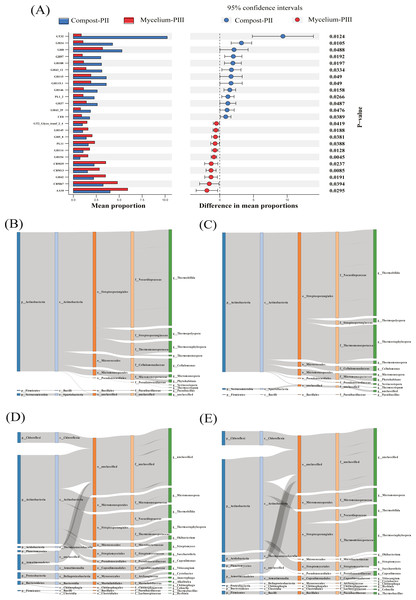
The effect of A. bisporus mycelium on the compost substrate was mainly reflected in the enzyme families with low relative abundance at the population level. For instance, the abundances of GT32 and GH24 significantly decreased, whereas those of GH42, AA10, and CBM13 significantly increased. Furthermore, the abundance of GH8 presented a slight decrease (Fig. 5A). GH24 is known to act in association with lysozyme, GH8 is the main family of enzymes involved in cellulose degradation, and GH42 plays an important role in hemicellulose degradation. Thermostaphylospora belonging to Actinobacteria mainly causes an increase in the activities of β-galactosidase and α-L-arabyranosidase of the GH42 family in the galactose metabolism pathway during the mycelial growth stage, and CBM67 has been reported to exhibit α-L-rhamnose-binding activity (Fujimoto et al., 2013).
Figure 5: Differentiated analysis of enzyme families.
(A) Difference of putative carbohydrate-activite enzymes of two groups samples. (B) Sankey plots for AA10 in compost-PII. (C) Sankey plots for AA10 in mycelium-PIII. (D) Sankey plots for cbm13 CBM13 in compost-PII. (E) Sankey plots for CBM13 in mycelium-PIII.In the present study, the increase in the laccase content during the mycelial growth stage was mainly related to the AA10 family (Table 2). The major strains that cause differential laccase production are Thermobifida, Thermostaphylospora, and Cellulomonas, and in the present study, the relative abundances of Thermobifida and Cellulomonas decreased, while that of Thermostaphylospora increased (Figs. 5B & 5C). Furthermore, the increase in the xylanase content during A. bisporus mycelial growth stage was mainly related to the CBM13 family, and Thermostaphylospora was also the dominant genus that caused this increase (Figs. 5D & 5E).
Discussion
Degradation of lignin during the composting process has been reported to be caused by certain fungi and several species of bacteria and Actinomycetes (Kabel et al., 2017; Fermor & Wood, 1981; Del Cerro et al., 2021; Ayuso-Fernández, Ruiz-Dueñas & Martínez, 2018). In the present study, the artificial introduction of A. bisporus at the end of the composting process and the resultant dominance of A. bisporus in the fungal community played a major role in lignin degradation. A similar finding has also been reported by Jean-Michel Savoie et al. (Savoie, 1998; Zhang et al., 2019). During the mycelial growth of A. bisporus, the oxidative phosphorylation pathway became dominant, which subsequently affected the bacterial and fungal communities composition, and a part of lignin degradation originated from bacterial action. Jurak, Kabel & Gruppen (2014) showed that xylan solubility increased by 20% during mycelial growth, indicating partial degradation of the xylan skeleton. In the present study, xylan degradation was mainly associated with the action of Thermostaphylospora. However, despite xylan degradation, the carbohydrate composition and degree of substitution of xylan in the compost at the beginning and end of the mycelial growth stage were rather similar (Jurak, Kabel & Gruppen, 2014). The protease activity that was measured had an increasing trend. This might imply that after accessing the mycelium of Agaricus bisporus, the raw material chicken manure and wheat straw were used for the breakdown of the macromolecular nitrogen source material and subsequently for the development and growth of their mycelium (Wang et al., 2021a and Wang et al., 2021b).
In a previous study, some researchers (Zhang et al., 2014) determined the rRNA gene copy number of Bacteria and Fungi during the composting process by quantitative PCR, and found that the fungal genus Agaricus and unknown fungal community accounted for 45% and 55% of the microbial community, respectively, while the bacterial genus Streptomyces accounted for 60% of the total bacterial community during the mycelial growth phase. In contrast, in the present study, although the abundance of Streptomyces varied before and after the mycelial growth stage, the difference was not significant. Moreover, besides Agaricus, Basidiobolus and Spizellomyces were also the predominant fungi, thus providing further insights into the composition of the fungal community in the compost.
During the A. bisporus mycelial growth stage, the relative abundances of gene sequences still remained high at high-temperature composting, and although the abundance of fungal communities increased, the number of bacterial genes was much higher than that of fungal genes. However, as it was not possible to determine the number of active bacteria during this stage, microbial communities with a higher relative abundance of gene sequences were compared, and A. bisporus was found to be dominant in the fungal community. While enzymes related to lignocellulose breakdown were not detected in the macrogenome of A. bisporus, analysis of whole-genome data of A. bisporus substrate confirmed the presence of a large number of genes encoding lignocellulose-degrading enzymes (Morin et al., 2012), because macrogenome sequencing results did not assemble genes encoding lignocellulose-degrading enzymes in A. bisporus. Moreover, variations were also noted in the expression of genes encoding CAZymes between compost-grown mycelia and fruiting body, with genes encoding plant cell wall degrading enzymes detected in compost-grown mycelia, but largely undetected in the fruiting body. Similarly, Patyshakuliyeva et al. (2013) also confirmed that compost-grown mycelia could express a large variety of CAZyme genes related to the degradation of plant biomass components. In addition, transcriptomics and proteomics investigations performed in a previous study also demonstrated that genes related to lignin degradation were only highly expressed on day 16 of mycelial growth, indicating that lignin was degraded at the initial stage of mycelial growth and was no longer altered after complete growth of mycelia (Patyshakuliyeva et al., 2015). Moreover, compost-grown mycelia were found to express a large number of CAZymes-encoding genes associated with the degradation of plant biomass components. In summary, the present study uncovered lignocellulose-degrading microorganisms and enzyme expressions in bacteria during A. bisporus mycelial growth stage in the composting process, providing further insights into lignin degradation in compost. Lignin degradation was mostly bacteria, and the main laccase-producing bacteria belonged to Thermobifida (Mironov et al., 2021; Vanee, Brooks & Fong, 2017) and Thermostaphylospora, with Thermostaphylospora presenting a significant increase. The results obtained can further strengthen our understanding of the specificity of A. bisporus mycelial growth.
Conclusions
The present study found the laccase content was increased during the A. bisporus mycelial growth stage. Laccases belonging to the AA10 family were mainly derived from Thermobifida and Thermostaphylospora, the potential for lignin-degrading enzymes of bacterial origin may be grossly underestimated. Agaricus proliferation may require an interacting consortium of both bacteria and fungi for effective lignocellulose degradation. The results obtained offer insights into the difference in enzyme activities between A. bisporus mycelia and other microbial communities, and enhance our understanding of the changes in microbial communities and enzyme families during A. bisporus mycelial growth phase in the composting process.
Supplemental Information
Physicochemical properties of Compost-PII and Mycelium-PIII
Notes. T1,T2,T3: different trials; Results represent mean ± standard deviation (n = 3).