Assessing lead and cadmium tolerance of Chenopodium ambrosioides during micropropagation: an in-depth qualitative and quantitative analysis
- Published
- Accepted
- Received
- Academic Editor
- Ravinder Kumar
- Subject Areas
- Agricultural Science, Biotechnology, Plant Science
- Keywords
- Chenopodium ambrosioides, Heavy metal, In-vitro culture, Phytoremediation, Tolerance
- Copyright
- © 2023 Jan et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Assessing lead and cadmium tolerance of Chenopodium ambrosioides during micropropagation: an in-depth qualitative and quantitative analysis. PeerJ 11:e16369 https://doi.org/10.7717/peerj.16369
Abstract
The tolerance of Chenopodium ambrosioides to some heavy metals under in vitro environment was thoroughly investigated. A micropropagation protocol was developed to facilitate the mass production of plants and to identify metals-tolerant species for potential use in the restoration of polluted areas. Nodal explants exhibited callus formation when treated with N6-benzyladenin (BA) (1.5 mg/l) and a combination of BA/α-naphthalene acetic acid (NAA) at concentrations of 1.5/1.0 mg/l on the Murashige and Skoog (MS) medium. The optimal shoot formation was achieved with the callus grown on a medium enriched with 1.5/1.0 mg/l BA/NAA, resulting in an impressive number (21.89) and length (11.79 cm) of shoots. The in vitro shoots were rooted using NAA (1.0 and 1.5 mg/l) and were acclimatized in pots with 71% survival rate. After standardizing micropropagation protocol, the in vitro shoots were subjected to various doses of lead nitrate (Pb(NO3)2 and cadmium chloride (CdCl2). Pb(NO3)2 and CdCl2 in the media let to a reduction in shoot multiplication, decreasing from 18.73 in the control group to 11.31 for Pb(NO3)2 and 13.89 for CdCl2 containing medium. However, Pb(NO3)2 and CdCl2 promoted shoot length from 5.61 in the control to 9.86 on Pb(NO3)2 and 12.51 on CdCl2 containing medium. In the case of Pb(NO3)2 treated shoots, the growth tolerance index (GTI) ranged from117.64% to 194.11%, whereas for CdCl2 treated shoots, the GTI ranged from 188.23% to 264.70%. Shoots treated with high level of Pb(NO3)2induced reddish-purple shoots, while a low level of Pb(NO3)2 induced shoots displayed both green and reddish-purple colors in the same explants. In CdCl2 treated culture, the toxic effects were narrow leaf lamina, elongated petiole and a dark reddish purple coloration. These findings highlight the remarkable potential of C. ambrosioides to maintain growth and organogenesis even in the presence Pb(NO3)2 and CdCl2 on the MS medium, indicating a high degree of metal tolerance.
Introduction
Soil contamination by heavy metals remains a serious problem and has becoming a great concern due to its adverse effects on humans and ecosystem throughout the world (Briffa, Sinagra & Blundell, 2020; Alengebawy et al., 2021). In this sense, certain plants have developed the remarkable ability to grow in soil with metal pollutants, accumulating these metals as part of their ecophysiological response in metalliferous environments (Ahmad & Ashraf, 2011). Among the hazardous elements, cadmium and lead stand out as notorious heavy metals, exerting adverse effects on plant growth and development due to their toxic nature and widespread existence in the environment (Jaishankar et al., 2014; Jamla et al., 2021). The impact of these metals extends to the structural, physiological and morphological aspects of plants, leading to growth degradation, wilting and ultimately plant mortality (Daud et al., 2015).
Cadmium is a non-degradable contaminant (Abbas et al., 2017), swiftly taken up by plants and transported to their above ground part, where it accumulates (Yang et al., 2010). This induces multiple symptoms of toxicity in plants, including leaf burning, chlorosis, stunted growth to reduced nutrient absorption (Kahle, 1993), disruptions in plant water balance (Ullah et al., 2020), and inhibition of stomatal opening (Bajguz & Hayat, 2009). Moreover, cadmium can disrupt chlorophyll synthesis, resulting leaf necrosis (Elazab, Abdel-Wahab & El-Mahdy, 2021) as well as reduce stem length and dry weight of roots in several plants (El-Banna & Abdelaal, 2018). The uptake and absorption of cadmium in plants pose a serious health risk to humans through the food chain (Shah & Dubey, 1998). Lead is among the toxic heavy metals (Zhang, 2003), occurring naturally in soil but significantly augmented by anthropogenic activities (Seregin & Ivanov, 2001).
Plant readily uptake lead from the soil, accumulating it in various plant parts thereby induce stress and other side-effects, such as reduced root elongation and biomass, inhibition of the chlorophyll synthesis, and impairment of certain enzyme activities (Fargasova, 1994; Miranda & Ilangovan, 1996). The plant’s response to lead contamination can vary, depending on the quantity present and the plant genotype exposed to the contaminant. Plant species selectively regarding lead uptake, accumulation ability, and tolerance to lead toxicity may differ (Janmohammadi, Bihamta & Ghasemzadeh, 2013; Topal et al., 2017). Plants contaminated with heavy metals can enter the animal food chain affecting animal meat and milk quality, and subsequently impacting human food (Caglarırmak & Hepcimen, 2010).
C. ambrosioides (L.) is an annual shrub belonging to the Chenopodiaceae family, graces the botanical landscape and reaches a maximum height of one meter (≤ 3 ft). This ecologically and medicinally important plant is commonly known as Mexican tea (referred to locally as Skhabotai in northwest Pakistan), and emits a distinctive, albeit unpleasant, aroma. Numerous studies have demonstrated that C. ambrosioides is rich in flavonoids and terpenoids, endowing it with diverse pharmacological properties, including antioxidant and cancer chemopreventive effects (e.g., Reyes et al., 2019; Kiuchi et al., 2002; Liu, 2004). Additionally, it has exhibited increased antioxidant activity in response to Cu-toxicity (Boojar & Goodarzi, 2007). Furthermore, an assessment of the n-hexane extract of C. ambrosioides has revealed molluscicidal activity (Hmamouchi, Lahlou & Agoumi, 2000). Nonetheless, the uncontrolled and excessive exploitation of medicinal plants including C. ambrosioides for culinary and medicinal purposes in many regions has raised conservation concerns (Chen et al., 2016; Rios et al., 2017; Khan et al., 2023). Likewise, the combined pressures of overexploitation along with the effect of climate change could potentially lead to a decline in the population of C. ambrosioides, ultimately threatening its availability for future generations. Thus, it becomes imperative to establish an effective regeneration system to protect the population size of C. ambrosioides to ensure its long-term sustainability. The current study presents the first effort to develop an innovative protocol for in-vitro propagation of C. ambrosioides through callus culture. Additionally, our aim is to assess the plant’s response to lead and cadmium stress using in-vitro culture as a well-controlled experimental system.
Materials & Methods
Experimental materials
Axillary branches measuring 7–9 cm in length were carefully excised from healthy C. ambrosioides plants growing at Malakand University in Khyber Pakhtunkhwa (KP), Pakistan. These branches were subjected to a meticulous cleansing process, involving a through rinsing with tap water for 6–7 min, followed by immersion in a solution of mercuric chloride (0.05%) containing few drops of Tween-20 for 12–15 min. Subsequently, the explants were washed with sterilized distilled water and the surface sterilized explants (2–3 cm long) were then horizontally cultured in screw capped jars containing Murashige & Skoog (1962) medium, enriched with 3% sucrose. The medium was semi-solidified with agar (0.6%), and its pH was meticulously adjusted to fall within the range of 5.50–5.56. Following the adjustment, the entire setup was adjusted to autoclaving at a temperature of 121 °C under a pressure of 15 psi for 10–13 min. To provide optimal growth, all cultures were incubated within a growth chamber at a constant temperature of 26 ± 2 °C, under a lighting system that simulated a 16-hr day followed by an 8-hr night cycle.
Callus induction and subculture for shoot initiation
Explant segments, comprising nodes, leaves, and petioles with sizes ranging from 2 to 3 cm, were placed in culture media containing varying concentrations of NAA (0.5, 1.0, and 1.5 mg/l), Indole-3-acetic acid (IAA) (0.5, 1.0, and 1.5 mg/l), and BA (0.5, 1.0, and 1.5 mg/l). These components were either used individually or combined with NAA (0.5 mg/l) or IAA (0.5 mg/l) to initiate the formation of callus (Table 1). After a thirty-day period, calluses that had developed from nodes, leaves, and petioles were isolated and subsequently transferred to growth media containing different concentrations of NAA (1.0, 1.5, and 2.0 mg/l), BA (1.5 and 2.0 mg/l), and combinations of BA/NAA (1.5/1.0 and 2.0/1.0 mg/l). These conditions were utilized to induce the formation of shoots through sub-culturing.
| PGR | Node | Leaf | Petiole | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Conc (mg/l) | %Re. | AC (mg) | Characteristic | %Re. | AC (mg) | Characteristics | %Re. | AC (mg) | Characteristics | |
| NAA | 0.5 | 57.46b | 75 | Reddish purple, brownish, compact, granular | 62.46b | 82 | Reddish purple, granular, soft | 58.46c | 102 | Creamy, Brownish, soft, granular |
| 1.0 | 63.82a | 138 | 57.82c | 163 | 73.82a | 156 | ||||
| 1.5 | 65.27a | 127 | 76.37a | 151 | 78.27a | 135 | ||||
| IAA | 0.5 | 47.32b | 67 | Light Reddish purple, soft, granular | 54.73c | 69 | Reddish purple, soft, Brownish, granular | 51.21c | 97 | Dark brownish, soft, granular |
| 1.0 | 52.93b | 122 | 64.78b | 138 | 68.32b | 175 | ||||
| 1.5 | 64.02a | 131 | 68.21b | 152 | 64.72b | 179 | ||||
| BA | 0.5 | 40.23c | < | Light yellowish compact, granular, light, hard | – | – | – | – | – | – |
| 1.0 | 45.18b | < | – | – | – | – | ||||
| 1.5 | 51.72c | < | – | – | – | – | ||||
| BA/ NAA | 0.5/0.5 | 57.38c | 102 | Light yellowish, friable, granular | 66.35b | 113 | Creamy, Reddish purple, granular, soft | 53.28c | 121 | Creamy, brownish, soft and friable |
| 1.0/0.5 | 64.71a | 162 | 58.32c | 159 | 65.16b | 189 | ||||
| 1.5/0.5 | 59.85b | 157 | 58.72c | 192 | 73.38a | 198 | ||||
| BA/IAA | 0.5/0.5 | 49.86b | 86 | Reddish purple, soft, granular | 47.83d | 95 | Light Reddish purple, soft, granular | 58.29c | 87 | Light brownish, soft, granular |
| 1.0/0.5 | 72.31a | 158 | 67.46b | 179 | 66.97b | 167 | ||||
| 1.5/0.5 | 60.39a | 149 | 62.91b | 173 | 73.31a | 183 | ||||
| Control | 0.0/0.0 | – | – | – | – | – | – | – | – | – |
Notes:
- PGR
-
Plant growth regulators
- Re
-
Response
- AC
-
Amount of callus
- <
-
less than 55
- –
-
Zero
Mean followed by different letters are statistically significant at p<0.05.
Shoot proliferation and root induction
For shoot proliferation, the in-vitro shoots were sliced into two to three pieces and transferred onto a growth medium supplemented with varying concentrations of growth regulators. Specifically, concentrations of 1.0, 1.5, and 2.0 mg/l of BA and kinetin were used. These growth regulators were applied either individually or in combination with NAA at a concentration of 1.0 mg/l. The aim was to standardize the concentrations and combinations for shoot proliferation (Table 2). The observation took place four weeks after the initial inoculation, where the shoot multiplication percentage, as well as the number and length of shoots, were recorded.
| PGR | Conc. (mg/l) | Node derived Callus | Leaf derived Callus | Petiole derived callus | |||
|---|---|---|---|---|---|---|---|
| NS ± Sd | LS (cm) ± Sd | NS | LS | NS | LS | ||
| NAA | 1.0 | 6.25 ± 0.61c | 3.21 ± 0.39a | NR | NR | NR | NR |
| 1.5 | 6.39 ± 0.34c | 4.04 ± 0.78a | ” | ” | ” | ” | |
| 2.0 | 5.45 ± 1.73c | 2.56 ± 0.26a | ” | ” | ” | ” | |
| BA | 1.5 | 10.84 ± 1.45a | 5.21 ± 0.83b | ” | ” | ” | ” |
| 2.0 | 14.83 ± 0.95b | 5.42 ± 0.08b | ” | ” | ” | ” | |
| BA/NAA | 1.5/1.0 | 9.43 ± 0.63a | 3.73 ± 0.29a | ” | ” | ” | ” |
| 2.0/1.0 | 8.78 ± 0.49a | 3.34 ± 0.84a | ” | ” | ” | ” | |
Notes:
- NS
-
Number of shoots
- LS
-
Length of shoots
- NR
-
Not responding
- ± Sd
-
Standard deviation
Mean followed by different alphabets are statistically significant at p < 0.05.
Moving on to root induction, individual shoots that were 30 days old were carefully excised and transferred to a medium containing a combination of BA and NAA at concentrations of 1.5/1.0 mg/l for the purpose of regeneration. Subsequently, these shoots were further placed on media supplemented with NAA at concentrations of 1.0, 1.5, and 2.0 mg/l. This was done both individually and in combination with BA/NAA at 1.5/1.0 mg/l and kinetin/NAA at 1.5/1.0 mg/l. The intention here was to facilitate the induction of roots. The progress of root formation, including induction percentage, numbers, and length, were then observed and recorded on the 24th and 26th days post-inoculation.
Acclimatization
Plantlets were gradually acclimated to a non-sterile environment by gently loosening the screws of the culture containers and lastly detached the cap. The plantlets were detached from the culture media and rinsed using sterilized distilled water, effectively eliminating the residual observing medium. These cleansed plantlets were then transplanted into plastic cups filled with a sterilized mixture of soil and sand (2:1 ratio). To initiate this transition, the plantlets were initially enclosed with plastic cups and wetted with half MS medium.
Heavy metals treatments and growth tolerance index (GTI)
In the context of heavy metals treatments, the in-vitro shoots were transplanted onto a growth medium enriched with specific metals, in conjunction with the optimal blend of BA/NA at a ratio of 1.5/1.0 mg/l. A series of concentrations for Pb(NO3)2 (0.5, 1.5, and 2.5 mg/l) and CdCl2 (0.5, 1.5, and 2.5 mg/l) were employed, combined with the previously mentioned optimal BA/NAA ratio (1.5/1.0 mg/l). The purpose of this study was to identify strains that exhibited a degree of tolerance. As a control group, an MS medium was employed that contained the ideal BA/NAA ratio (1.5/1.0 mg/l), yet lacked the introduction of Pb(NO3)2 and CdCl2. Subsequent to these treatments, the in-vitro shoots underwent assessment in terms of measurements and weight determination. To ascertain the dry biomass, the plant materials underwent a 24-hour exposure to 80 °C and were subsequently weighed. This facilitated the computation of the growth tolerance index (GTI) for the shoots, presented as a percentage. The GTI was calculated using the following formula:
Statistical analysis
Differences among the treatments were assessed using a single factor analysis of variance (ANOVA). Furthermore, the variances among the variables were examined by a post-hoc Tukey honest significant difference (HSD) test. Statistically significant variations were considered significant at a threshold of P < 0.05. To assess the impact of heavy metals, we conducted a correlation analysis involving multiple parameters of C. ambrosioides such as the concentration of heavy metals (HMC mg/l), the growth tolerance index (GTI %), multiplication rate (%), number of shoots, shoot length, and biomass. All the statistical analyses were performed in XLstat and OriginLab Pro-2023b (https://www.originlab.com) software’s.
Results
Callus induction
Node, leaf and petiole of C. ambrosioides were inoculated on media containing various concentrations of NAA, IAA, BA, NAA/BA, IAA/BA and NAA/IAA as outlined in Table 1. Within a span of 10 days from inoculation, all three types of explants exhibited the development of callus. The texture of resulting callus varied, ranging from compact and soft to friable and granular. Specifically, callus formation occurred in petiole explant at a rate of 78.27%, leaf at a rate of 76.37% when treated with 1.5 mg/l NAA, and in node at a rate of 65.71% with 1.0/0.5 mg/l BA/NAA (Table 1). Notably, the callus developed from nodes, particularly under the influence of BA with or without presence of NAA, exhibited a remarkable organogenic potential. Conversely, the callus derived from petiole and leaf explants did not display any organogenic potential (Table 2). In the case of IAA, both alone and in combination with BA, the formation of callus was observed; however, their corresponding fresh weights and percentage responses remained relatively low (Table 1). Explants that were placed on a growth regulator-free medium (control) failed to exhibit any growth and eventually died after 15 days of inoculation.
Shoot from callus
Thirty days old callus from node, leaf and petiole were isolated and sub-cultured on media containing NAA, BA and BA/NAA (Table 2). Nodal explants produced compact, friable, glandular callus with a light yellowish type. In the presence of BA, either alone or with conjunction with NAA (Table 2), these nodal callus cultures displayed prolific shoot formation. The average number of shoot formed with BA ranged from 10.84 ± 1.45 to 14.83 ± 0.93, with corresponding shoot lengths ranging from 5.21 ± 0.83 to 5.42 ± 0.83. Conversely, when utilizing the combination of BA/NAA, the average number of shoots formed ranged from 9.43 ± 0.63 to 8.78 ± 0.49. Notably, the application of NAA (at concentration of 1.0–2.0 mg/l) resulted in the formation of an average of 6.25 ± 0.61 to 5.45 ± 1.73 shoots, respectively. Nevertheless, callus derived from leaf and petiole sources exhibited a lack of shoots regeneration (Table 2).
Multiplication of shoots
Cluster of shoots (2–3) were sub-cultured on media having various concentrations and combinations of BA, Kn or BA/NAA and Kn/NAA in order to standardized the optimal concentration and combination for shoot multiplication (Table 3). The results of shoots multiplication were recorded for all concentrations of BA and Kn, both with or without NAA (Table 3). Notably, the application of BA/NAA induced the highest number of shoots, ranging from 21.89 ± 1.94 to 18.39 ± 1.68, with their shoot lengths ranged from 5.49 ± 0.84 to 4.82 ± 0.76 cm. The supplementation of BA alone proliferated shoots in the ranged of 16.5 ± 1.03 to 14.61 ± 0.61. Interestingly, Kn either alone or in combination, produced less number of shoots compared to BA, whether applied separately or in combination (Table 3).
| PGR | Concentration (mg/l) | Number of shoots ± Sd | Length of shoots (cm) ± Sd |
|---|---|---|---|
| BA/NAA | 1.0/1.0 | 18.73 ± 1.67a | 5.26 ± 0.27a |
| 1.5/1.0 | 21.89 ± 1.94a | 5.49 ± 0.84a | |
| 2.0/1.0 | 18.39 ± 1.68a | 4.82 ± 0.76a | |
| BA | 1.0 | 16.57 ± 1.03b | 3.65 ± 0.72b |
| 1.5 | 16.49 ± 0.82b | 4.05 ± 0.32b | |
| 2.0 | 14.61 ± 0.61b | 3.95 ± 0.84b | |
| Kn/NAA | 1.0/1.0 | 11.43 ± 0.72c | 3.13 ± 0.39c |
| 1.5/1.0 | 9.87 ± 0.54c | 3.47 ± 0.48c | |
| 2.0/1.0 | 9.35 ± 0.39c | 2.78 ± 0.07c | |
| Kn | 1.0 | 7.83 ± 0.86c | 2.58 ± 0.09c |
| 1.5 | 7.95 ± 0.35c | 2.47 ± 0.41c | |
| 2.0 | 8.46 ± 0.08c | 1.85 ± 0.15c | |
| Control | – | – | – |
Notes:
Root induction
The best rooting rate (76.73%) and number (12.32 ± 0.58) were recorded in the medium comprising NAA at a concentration of 1.0 mg/l. The formation of roots with BA/NAA (1.5/1.0 mg/l) resulted in a lower number (8.07 ± 0.16) but higher length (13.23 cm). However, the medium supplemented with Kn/NAA did not support roots formation (Table 4).
| PGR | Concentration (mg/l) | % Rooted shoot | Roots number/ explant ± Sd | Roots length (cm)± Sd |
|---|---|---|---|---|
| NAA | 1.0 | 76.73a | 12.32 ± 0.58a | 9.34 ± 0.42b |
| 1.5 | 68.02b | 11.61 ± 0.47a | 12.81 ± 0.72a | |
| 2.0 | 64.89b | 9.89 ± 0.09b | 10.79 ± 0.91b | |
| BA/NAA | 1.5/1.0 | 40.21c | 8.07 ± 0.16b | 13.23 ± 1.03a |
| Kn/NAA | 1.5/1.0 | – | – | – |
Notes:
Acclimatization
The survival potential of the in-vitro regenerated plantlets when transferred to the ex-vitro environment was 71% (Fig. 1). Notably, there were no obvious phenotypic differences observed between the acclimatized plants and the original mother stock. Subsequently, the two month old plantlets were then transplanted into the lath house.
Figure 1: In-vitro propagation of C. ambrosioides.
(A) Shoots from callus, (B) abnormal shoots with BA/CdCl2, (C) abnormal and normal shoots with BA/Pb(NO3)2, (D) normal shoots with BA/NAA, (E) roots with NAA, (F) acclimatized plant.Heavy metals and shoot proliferation
After optimizing of culture condition, subsequent experiments were designed to identify a suitable line for metals (Pb(NO3)2 and CdCl2) tolerance (Table 5). The inclusion of Pb(NO3)2 in the medium inhibited shoots multiplication rate. The percentage of shoot multiplication per explants dropped from 86.74% in the control treatment to 57.38% in culture treated with Pb(NO3)2 (Table 5). Although, Pb(NO3)2 containing media did not exhibit any visible symptoms of phenotypic alteration except for a reddish purple color, all shoots maintained a normal morphology characterized by broad lamina and short petiole similar to those of the mother plant. In some instances, the same explants produced both normally morphed shoots with green and reddish purple color shoots (Fig. 1C) at 0.5 mg/l of Pb(NO3)2 in the medium. The growth of the shoot biomass (dry weight) remained unaffected by Pb(NO3)2 at 0.5 mg/l in the medium and was comparable to the control, while higher concentration Pb(NO3)2 in the medium led to an increase biomass growth (Table 5).
| Metals (mg/l) | Multiplication % | Number of shoots ± Sd | Length of shoot ± Sd | Toxicity symptoms | Shoots dry weight (mg)± Sd | |
|---|---|---|---|---|---|---|
| Lead | Cadm. | |||||
| 0.5 | 57.38 | 14.57 ± 2.03a | 5.16 ± 1.29c | In few illustrations green and reddish purple branches with normal leaf and petiole were noted. | 20 ± 1.8a | |
| 1.5 | 68.71 | 11.69 ± 2.82b | 7.52 ± 1.44b | Shoots were reddish purple color with broad leaf lamina and short petiole as in the mother plants. | 26 ± 2.3b | |
| 2.5 | 59.85 | 11.31 ± 1.61b | 9.86 ± 2.36a | 33 ± 2.9b | ||
| 0.5 | 75.71 | 16.31 ± 1.61a | 6.36 ± 1.47c | Normal | 32 ± 2.08b | |
| 1.5 | 63.38 | 13.89 ± 1.94b | 8.15 ± 1.39b | Reddish purple color shoots with disturbed morphology (narrow leaf lamina and elongated petiole) | 37 ± 2.6b | |
| 2.5 | 59.25 | 14.09 ± 1.68b | 12.51 ± 2.73a | 41 ± 3.35b | ||
| Control | 86.74 | 18.73 ± 1.67a | 5.61 ± 1.46c | Normal | 17 ± 2.0a | |
Notes:
The culture of C. ambrosioides vigorously reproduced in-vitro shoots proliferation in media supplemented with CdCl2. The highest propagation percentage (75.71%) was obtained with 0.5 mg/l of CdCl2 concentration in the medium. The shoots formed under varying concentrations of CdCl2 ranged from 16.31 ± 1.61 to 13.89 ± 1.94 per explants. Notably, in presence of lower CdCl2, concentrations, shoot multiplication was as high as in the control (Table 5). Shoots with 0.5 mg/l CdCl2 displayed a normal morphology, while 1.5 and 2.5 mg/l of CdCl2 induced morphological abnormalities in the in-vitro shoots. The abnormalities included narrow leaf lamina, elongated petioles, and reddish purple coloration (Fig. 1B). Biomass growth of shoots exhibited an increased trend with increasing concentration of CdCl2 (Table 5).
Heavy metals and growth tolerance
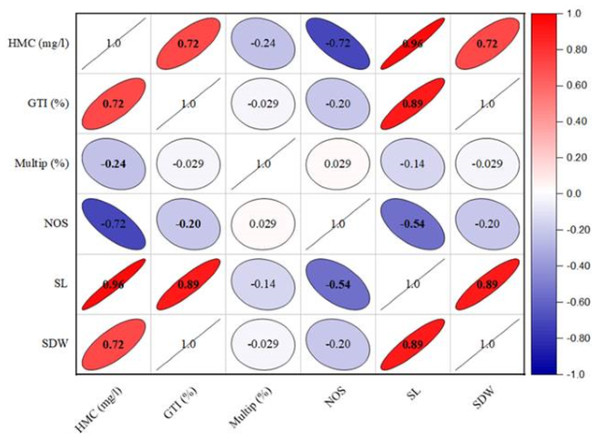
The growth tolerance index (GTI) calculation revealed that shoot growth was enhanced in the presence of heavy metals (Table 6). The highest GTI value was observed in the medium comprising 2.5 mg/l of lead, reaching to 194.11%. Conversely, lower doses of lead resulted into low dry biomass production, with GTI values of 152.94% for 1.5 mg/l and 117.64% for 0.5 mg/l. In contrast, the GTI exhibited a reduction in culture grown on cadmium-containing medium compared to lead-containing medium. The GTI values for cadmium-containing media ranged from 188.23% to 264.70% as given in Table 6. Furthermore, the heavy metals concentration has a significant positive (r = 0.671; p < 0.01) effect on GTI (%), shoot length (r = 0.924; p < 0.001) and dry weight ((r = 0.651; p < 0.01) respectively (Fig. 2). In contrast, the HMC (mg/l) presence in the media exhibited an adverse effect on multiplication (r = − 0.24; p < 0.05) and number of shoots (r = − 0.655; p < 0.01).
| Treatment | (GTI)(%) | |
|---|---|---|
| Lead | Cadmium | |
| 0.5 | 117.64 | |
| 1.5 | 152.94 | |
| 2.5 | 194.11 | |
| 0.5 | 188.23 | |
| 1.5 | 217.64 | |
| 2.5 | 264.70 | |
Figure 2: The effect of heavy metals concentration on various structural attributes of C. ambrosioides.
Discussion
Plant tissue culture provides several advantages for studying the metabolic capabilities of plant cells and their capacity for toxicity tolerance. Understanding the impacts of plant cells on pollutant uptake and purification, free from microbial interference, hold significant importance in the pursuit of essential knowledge about plants (Doran, 2009). This study aimed to establish an effective in vitro protocol for the large-scale multiplication of C. ambrosioides through callus culture. Additionally, it sought to assess the tolerance ability of plant material to lead and cadmium stress using aseptic in-vitro culture techniques. In our studies, the morphogenic responses of the three types of explant showed variation based on the concentration and combinations of growth regulators employed. Nodal explants exhibited the development of multiple shoots within the callus, while leaf and petiole explants proved conducive to callus formation. Prior research, such as the study conducted by Hesami & Daneshvar (2016) on C. quinoa, as well as Nathar & Yatoo (2014) on Artemisia pallens, highlighted the superior suitability of hypocotyle for C. quinoa and shoot tip, leaf, and petiole for Artemisia pallens as explants for callus formation. Our study contributes by documenting the formation of compact, friable, granular callus with a light yellowish type from node, leaf and petiole explants under the influence of either BA alone or in combination with NAA. It is north noting, that unlike the nodal explant, the callus derived from leaf and petiole has not been able to demonstrate organogenic potential.
The difference in shoots formation between different types of explants in response to BA could be linked to the level of endogenous cytokinins as suggested by Yucesan, Turke & Gure (2007). Our results exposed that, in the case C. ambrosioides, BA exhibited its cytokinin activity most effectively at lower concentration, facilitating multiplication of new shoots. However, as the concentration of BA increased, the numbers of newly formed shoots show a decrease. This observation aligns with the study of Marino et al. (1993) which highlighted a similar trend in apricot shoots growth. Specifically, they noted an increased in shoot numbers with increasing BA levels up to 2.0 mg/l, beyond which a subsequent rise in concentration led to a reduction shoot multiplication. For achieving optimal shoots multiplication in our study, a moderate concentration of cytokinin (BA) at 1.5 mg/l and a low level of auxin (NAA) at 1.0 mg/l were found to be necessary.
Morphologically, the shoots that developed under this specific combination exhibited normal characteristics including green shoots, well-formed leaves and healthy petioles. The significance of utilizing a combination of growth regulators has been emphasized in several studies. Raihana et al. (2011) produced the maximum number of shoot proliferation in Curcuma manga through the application of BA and NAA, while Bharalee, Das & Kalita (2005) achieved similar results in Curcuma caesia. In our root induction experiments, the in-vitro shoots of C. ambrosioites also displayed root formation on the same medium (BA/NAA) used for shoot multiplication. Additionally, roots were successfully induced using media containing varying concentration of NAA. These results are consistent with the outcomes reported by Mongkolsawat et al. (2018) and Jan et al. (2020).
Several researchers aspire to provide essential nutritious food and fodder for the future (White, 2012). But ensuring the safety of both food and fodder is of paramount importance. The toxic properties of Cd have been extensively studied and ongoing research is now centered on the cultivation of crops that can withstand Cd presence in the environment. Additionally, efforts have been made to cultivate plants with the capacity to accumulate Cd for purpose phytoremediation in polluted lands (White, 2012; Martinka, Vaculík & Lux, 2014; White & Pongrac, 2017). The achievement of successful phytoextraction is commonly linked to a plant’s capability to endure the presence of of pollutant in the substrate (Di-Lonardo et al., 2011).
To determine the tolerance ability, the in-vitro shoots of C. ambrosioides were exposed to different concentrations of lead and cadmium. In the eco-toxicological evaluation, it is essential to avoid lethal doses of the metals toxicity in order to select the tolerant line, its concentration has to be appropriately raised (Wierzbicka et al., 2007; Doran, 2009). Considering the observed tolerance of the evaluated C. ambrosioides, it can be concluded that there was no shoot necrosis on any media containing lead or cadmium. This absence of necrosis suggests the evaluated concentrations of metals did not indicate pollution which has also been advocated by Katanic et al. (2007). In our experiments, the application of lead treatment resulted in a notable suppression of the proliferation percentage, leading to a reduction of 57.38% at 0.5 mg/l concentration. Conversely, at the same concentration (0.5 mg/l), cadmium exposure triggered a proliferation rate of 75.71%. Interestingly Alyssum montanum exhibited enhanced multiplication, rooting, and biomass production at low to moderate levels of cadmium, as observed by Wiszniewska et al. (2017).
The encouraging impact of lead has also been reported by Seregin & Ivanov (2001) on root multiplication. The increase in shoot length was observed across all concentrations of Pb and Cd in the growth media; however the positive effect of Cd was more pronounced compared to Pb. In our research, the increase in shoot length and dry biomass served as useful indicators for assessing metal tolerance in plants. The phenomenon of metals working synergistically to promote plant growth was similarly reported by John et al. (2008). Kalisov-Spirochova et al. (2003) also noted a positive effect of Pb on total biomass accumulation in aspen rooted-shoots cultured in vitro. Nevertheless, Katanic et al. (2007) reported an adverse effect on the fresh biomass of white poplar shoot tips when grown on the multiplication medium enriched with Pb.
In our study, Pb and Cd induced phenotypic alteration in shoots at higher doses. Highest doses of Pb induced reddish purple color shoot while low dose induced green and reddish purple shoots from the same explant. It was observed, that high Cd stress induced greater toxicity and the visible toxic symptoms were narrow leaf lamina and short petiole and reddish purple coloration. Similar toxicity symptom has been reported by Pandey & Sharma (2002) in cabbage plant. The stunted growth rate in plants at high dose of cadmium may be because of a disorder in the enzymatic activities, transpiration and photosynthesis (Almeida et al., 2007). C. ambriosoides maintained biomass production on Pb and Cd selection system of shoots what can be considered an indicator of metals tolerance (Gomes, Marques & Soares, 2013). Comparing the tolerance index, C. ambriosoides tolerant to Cd concentrations compared to Pb. Our findings are accordance with the findings of other studies (e.g., Zacchini et al., 2009; Dos-Santos et al., 2007).
Conclusions
Plant tissue culture techniques, such as in vitro shoot cultures, play central role in phytoremediation studies. In the case of the studied C. ambrosioides, it exhibited a greater capacity to maintain growth and organogenesis in medium containing CdCl2 compared to Pb(NO3)2. The influence of cadmium on the plant’s development surpassed that of lead, resulting in enhanced shoot length, greater shoot dry weight, and notable phenotypic alterations within the in-vitro shoot structure. Thus, C. ambrosioides display a higher tolerance towards CdCl2 than Pb(NO3)2, as evidenced by its elongated shoots, total plantlet biomass, and GTI and may be used as a candidate plant for phytoremediation.