A novel gene silencing strategy based on tobacco rattle virus in Hibiscus mutabilis
- Published
- Accepted
- Received
- Academic Editor
- Vladimir Uversky
- Subject Areas
- Bioengineering, Biotechnology, Molecular Biology, Plant Science
- Keywords
- Virus-induced gene silencing, Tobacco rattle virus vector, Hibiscus mutabilis, Cloroplastos alterados 1, CLA1, Gene silencing
- Copyright
- © 2024 Sang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. A novel gene silencing strategy based on tobacco rattle virus in Hibiscus mutabilis. PeerJ 12:e18211 https://doi.org/10.7717/peerj.18211
Abstract
Background
Hibiscus mutabilis L. is a popular regional characteristic plant in China, cultivated for its attractive flower colors, extended bloom time, and medicinal properties. To enhance molecular breeding and gene function studies, we conducted transcriptome analysis and identified valuable genes in previous research. Nonetheless, the current inefficient and labor-intensive transformation techniques have hindered their applications. Virus-induced gene silencing (VIGS) provides a precise and effective strategy for post-transcriptional down-regulation of endogenous gene expression.
Methods
We investigated the performance of tobacco rattle virus (TRV) as a tool for targeting and silencing the gene encoding the protein involved in chloroplast development, cloroplastos alterados 1 (altered chloroplast; CLA1), of H. mutabilis through Agrobacterium tumefaciens-mediated infiltration.
Results
By effectively suppressing the CLA1 gene associated with chloroplast development in H. mutabilis via the TRV-VIGS system, we have illustrated the inaugural implementation of VIGS in this species. Quantitative RT-PCR proved that HmCLA1 expression in agro-infiltrated plants was lower than in the mock-infiltrated (mock) and the control (CK) plants. Phenotypic observations corroborated the albino phenotype in leaves following successful HmCLA1 silencing.
Conclusions
Our study showcases TRV-VIGS as a potential gene silencing tool for H. mutabilis, facilitating functional genomics studies and molecular breeding efforts in this species.
Introduction
Hibiscus mutabilis, also known as cotton rose, is a flowering shrub belonging to the genus Hibiscus in the family Malvaceae. It stands out as one of the most popular regional characteristic plants in China. H. mutabilis has been cultivated south of the Yangtze River for over 2,000 years and holds significant importance as the city flower of Chengdu. It is widely planted in public gardens and urban greenbelts due to its diverse flower colors, extended flowering duration, and floral development and morphogenesis (Yang et al., 2022). Besides, the leaves of cotton rose serve as an ingredient of traditional herbal remedies due to their blood cooling, detoxification, anti-inflammatory, and analgesic properties (Liu et al., 2015a, 2015b). To facilitate research on molecular breeding and gene function, we have identified numerous excellent genes through transcriptome analysis. However, the hard and inept plant transformation methods hamper the application of these findings to various species. It is extremely important that more suitable techniques be successfully employed to facilitate the investigation of plant gene roles (Wang et al., 2019).
Virus-induced gene silencing (VIGS) is an effective genetic method used to analyze the functions of a gene. It uses plant viruses engineered as tools or vectors to specifically decrease the endogenous expression of a gene via post-transcriptional gene silencing (PTGS) (Caplan & Dinesh-Kumar, 2006; Purkayastha & Dasgupta, 2009; Becker & Lange, 2010; Ramegowda, Mysore & Senthil-Kumar, 2014; Krishnan et al., 2015; Ashfaq et al., 2020). To begin with, VIGS was first employed in Nicotiana benthamiana to silence the gene encoding phytoene desaturase (PDS) (Kumagai et al., 1995). Subsequently, it has been adopted in several angiosperms, namely, Arabidopsis thaliana (Burch-Smith et al., 2006), cotton (Qu et al., 2012), tomato (Orzaez et al., 2009), wolfberry (Qu et al., 2021), and some woody plants (Jiang et al., 2014; Liu et al., 2014; Wang et al., 2019). The availability of numerous vector systems for VIGS in eudicot plants provides an improved possibility of constructing one for H. mutabilis. Double-stranded RNA (dsRNA) is a key factor in VIGS. Typically, the RNA interference (RNAi) pathway starts with the cleavage of dsRNA by DICER into 21- to 25-nucleotide-long short interfering RNAs (siRNAs) with two strands, the guide strand and the passenger strand (Lu et al., 2003; Burch-Smith et al., 2004; Jiang et al., 2014). The RNA-induced silencing complex (RISC) further identifies these siRNAs and causes the degradation of the passenger strand while using the guide strand to identify, incorporate, and degrade complementary single-stranded RNA in the cell (Mustafa et al., 2016). Consequently, the gene gets silenced, leading to the generation of substantial quantities of siRNAs (Fuchs, Damm-Welk & Borkhardt, 2004).
Tobacco rattle virus (TRV), belonging to the Tobravirus genus (Virgaviridae family), is a vector system suitable for VIGS (Jiang et al., 2014). Researchers have developed and applied TRV-mediated VIGS using Agrobacterium-based transformation in cotton (Gao et al., 2011; Mustafa et al., 2016; Ge et al., 2016; Shi et al., 2021). The TRV genome consists of RNA1 and RNA2, two positive-sense single-stranded RNAs. Among these, RNA1 (TRV1) encodes proteins associated with movement and replication, while RNA2 (TRV2) encodes the coat protein and a few nonessential structural proteins. And these nonessential structural proteins can be deleted to insert foreign sequences (Hayward, Padmanabhan & Dinesh-Kumar, 2011; Senthil-Kumar & Mysore, 2014; Mustafa et al., 2016). Besides, CLA1 is a highly conserved gene involved in chloroplast development across various plants, such as Arabidopsis (Mandel et al., 1996), and its mutant albino phenotype makes it a valuable marker for assessing silencing efficiency (Cheng et al., 2023).
Therefore, the present study employed the TRV-based system to silence the CLA1 gene in H. mutabilis. We developed the TRV vector system for gene targeting and infiltrated leaves of H. mutabilis, using TRV alone as the mock. Furthermore, we analyzed the phenotype and HmCLA1 expression in the leaves of the treated, mock, and control plants. The study’s findings will demonstrate the efficacy of the TRV system in silencing H. mutabilis genes via the VIGS approach. Importantly, this study represents the first successful use of VIGS in H. mutabilis, filling a crucial gap in gene silencing techniques and advancing the screening of functional genes and molecular breeding in H. mutabilis.
Materials and Methods
Plant material and growth conditions
The H. mutabilis cultivar ‘single-petal white’ was used. Seeds were collected from the Chengdu Botanical Garden. The seeds were subjected to a 15-min treatment with concentrated sulfuric acid and then washed thoroughly with sterile water. These seeds were then sown in pots filled with a peat and garden soil (v:v; 1:1) and maintained in an incubator at 25 °C controlled temperature, lux light, and a photoperiod of 14 h light/10 h dark. This study was performed in nursery of Chengdu Botanical Garden from June 2023 to May 2024.
Analysis of HmCLA1 sequence and construction of phylogenetic tree
Utilizing the HmCLA1 nucleotide sequence (GenBase accession no. C_AA041596.1) as a foundation, the inferred protein sequence was compared with the CLA1s from diverse species using the ClustalX2 software (Larkin et al., 2007). Furthermore, the amino acid sequences obtained from NCBI (https://www.ncbi.nlm.nih.gov/) were utilized to build a phylogenetic tree in MEGA 11. The Neighbor-Joining (NJ) method was used with 1,000 standard bootstrap replicates (Koichiro, Glen & Sudhir, 2021).
Construction of the VIGS vector
Total RNA was isolated from the H. mutabilis ‘single-petal white’ leaves using RNA-prep Pure (TIANGEN, Beijing, China) and reverse transcribed to cDNA using the HiScript II 1st Strand cDNA Synthesis Kit (+gDNA wiper) (Vazyme, Nanjing, China). Further, PCR was performed using this cDNA with the HmCLA1-F and HmCLA1-R primers (Table 1) to amplify a partial fragment of HmCLA1. These primers were designed by the DNAman 9.0 software for the conserved domain, with the EcoR I and BamHI sites incorporated into the upstream and downstream primers, respectively. PCR was conducted under the following thermal cycling conditions: 32 amplification cycles, including denaturation for 15 s at 95 °C, annealing for 15 s at 60 °C, and extension for 1 min at 72 °C. The reaction mixture, with a final volume of 50 µL, consisted of 2 × Phanta Max Master Mix (25 µL), the dNTP mix (1 µL; 10 mM each), primer (2 µL; 10 µM), cDNA (4 µL), and Phanta Max Super-Fidelity DNA Polymerase (1 µL; 1 U/µL). The pTRV1 and pTRV2 vectors were employed for vector construction (Ratcliff, Martin-Hernandez & Baulcombe, 2001). The amplicons were ligated into the EcoR I and BamHI double-digested pTRV2 vector with the ClonExpress II One Step Cloning Kit (Vazyme, Nanjing, China) to obtain the pTRV2-HmCLA1 construct.
| Primer name | Primer sequence |
|---|---|
| TRV-HmCLA1-F | gctcggtaccggatccCACAACATCGATGATTTAG |
| TRV-HmCLA1-R | ttaaggttaccgaattcATGATGAGTAGATTGCACA |
| qRT-HmCLA1-F | ATCGGCAAAGGAAGGGTACT |
| qRT-HmCLA1-R | CGTGGGATTCCAATAACGAG |
| qRT-HmActin-F | GATGAAGATTCTTACTGAGAG |
| qRT-HmActin-R | CAGAAGAGCTGCTCTTGGCAG |
Agroinfiltration
In the context of H. mutabilis, the pTRV2-HmCLA1 vector was initially introduced into Agrobacterium tumefaciens ‘GV3101’ cells by the freeze-thaw method (Höfgen & Willmitzer, 1988). Subsequently, a single colony confirmed by PCR was inoculated into 3 mL of Yeast Extract Peptone Broth medium containing rifampicin (25 mg/L), kanamycin (50 mg/L), and gentamicin (50 mg/L) and allowed to multiply in a shaker at 200 rpm and 28 °C for 12 h. Additionally, A. tumefaciens cells carrying the empty vector (pTRV1 or pTRV2) were cultured in 3 mL of the same media under identical conditions. These starter cultures were subcultured into 50 mL of fresh media and maintained at 28 °C for almost 16 h. For the ensuing assay, the Agrobacterium cultures were centrifuged at 6,000 × g for 10 min. The obtained pellets were then dissolved in a buffer (150 µM acetosyringone (AS), 10 mM MES, 10 mM MgCl2 (pH = 5.6)) to an optical density (OD600) of 2.0 and left undisturbed for 3 h at room temperature. Finally, the pTRV2-HmCLA1 and pTRV1 Agrobacterium cultures were mixed at a 1:1 ratio and infiltrated into the fully opened H. mutabilis cotyledons with a 1 mL needleless syringe. A mixture of Agrobacterium cells carrying pTRV1 and pTRV2 constructs in a 1:1 ratio was infiltrated into the cotyledons and used as mock plants. This procedure followed a previously established protocol (Gao et al., 2011).
Quantitative real-time PCR
Quantitative real-time PCR (qPCR) was carried out with the qRT-HmCLA1-F/qRT-HmCLA1-R primers (Table 1) to quantify the HmCLA1 transcripts (relative abundance) in the gene-silenced leaves with visible phenotypes, with the control group and mock group as references. HmActin and HmAFD2 were used as the reference gene for the assay. Three biological replicates were analyzed per group. Each biological replicate was subjected to three technical replicates. Total RNA extraction and reverse transcription refer to the method description provided earlier. The cDNA was then used to assess the transcript abundance performing qPCR with the AceQ qPCR SYBR Green Master Mix (Jizhenshengwu, Shanghai, China) on a CFX96 System (Bio-Rad, Hercules, CA, USA). PCR was conducted under the following thermal cycling conditions: one cycle of pre-denaturation for 5 min at 95 °C, 44 cycles of denaturation for 10 s at 95 °C, and annealing and extension for 30 s at 60 °C. The reaction mixture, with a final volume of 10 µL, consisted of 5 μl of 2xSYBR Green Mix, 0.5 μL of each Primer F+R (each 10 uM), and 2 µL of cDNA. Finally, the relative expression level of HmCLA1 was determined following the 2−ΔΔCt method (Livak & Schmittgen, 2001; Gu et al., 2018). We use standard deviation to measure the repeatability within groups, and we use a T-test to assess whether there is a significant difference between groups.
Statistical analysis
One-way analysis of variance (ANOVA) and Duncan’s multiple range test (P < 0.05) were performed using R (Version 4.3.2).
Results
Sequence analysis of HmCLA1 in H. mutabilis
Our research shows that the full-length HmCLA1 CDS is 2,166 bp, with the longest ORF encoding 721 amino acids. The predicted gene products of HmCLA1 were 77.23 KDa proteins with pI of 6.8 (https://web.expasy.org/protparam/). The forward primer used for vector construction spans bases 997 to 1,015, while the reverse primer spans bases 1,417 to 1,399. For qPCR detection of HmCLA1 expression, the forward primer spans bases 1,723 to 1,742, and the reverse primer spans bases 1,828 to 1,809 (Fig. S1). Additionally, we also performed CDS sequence alignments of CAL1 among the five species (Fig. S3).
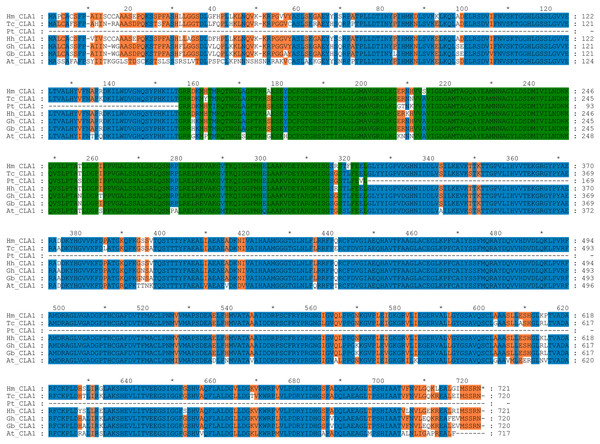
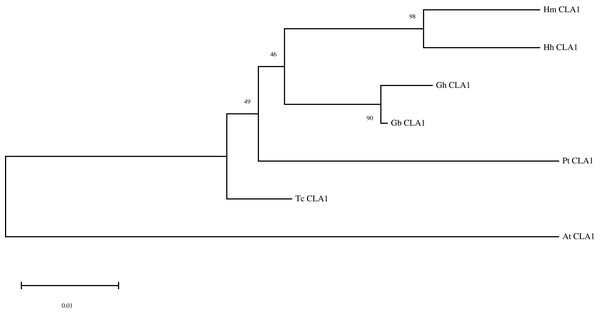
By performing multiple sequence alignments of CAL1 proteins, we identified a significant similarity between HmCLA1 and CLA1 from other plant species (Fig. 1 and Table S1). HmCLA1 and HhCLA1 formed a clade on the phylogenetic tree. This observation indicated that HmCLA1 shared a high level of similarity with HhCLA1 found in H. hamabo, followed by GhCLA1 and GbCLA1 from G. hirsutum and G. barbadense in the Malvaceae family (Fig. 2).
Figure 1: Multiple alignment of the HmCLA1 amino acid sequence with CLA1 amino acid sequences from different species using the ClustalW program.
Multiple alignment of protein sequences of the CLA1 gene in Hibiscus mutabilis, Hibiscus hamabo (MK229167), Gossypium barbadense (ABN13970.1), Gossypium hirsutum (NP_001314056.1), Theobroma cacao (EOY06359.1), Populus tomentosa (AGT02336.1), and Arabidopsis thaliana (NP_193291.1).Figure 2: Phylogenetic analysis of the protein of HmCLA1.
Phylogenetic analysis of CLA1 proteins in different species.HmCLA1 silencing using the VIGS approach
In this study, we inoculated seventy-one plants with A. tumefaciens ‘GV3101’ harboring pTRV2-HmCLA1, 9–12 days after the emergence of the two cotyledons. These plants showed no symptoms of infection in the initial 2 weeks. However, 18 days post-infection, white streaks appeared on the emerging leaves of the pTRV2-HmCLA1 agro-infiltrated plants. Ultimately, 74.65% of these plants displayed white-streak leaf symptoms resembling the photobleached phenotype (Table 2 and Fig. 3B). In contrast, no white streaks were found on the leaves of the mock and CK plants (Figs. 3A and 3C). Individual leaves of all infiltrated plants are shown in Fig. 3D. The observed phenotypic characteristics of the leaves suggested that plants infiltrated with pTRV2-HmCLA1 might experience suppression of HmCLA1 gene expression compared with mock and CK plants.
| Treatment | Number of plants assayed | Silencing efficiency* |
|---|---|---|
| pTRV2-HmCLA1 | 71 | 53/71 (74.65%) |
| Mock | 10 | 0/10 (0%) |
| Ck | 10 | 0/10 (0%) |
Note:
Figure 3: TRV-induced HmCLA1 silencing in H. mutabilis.
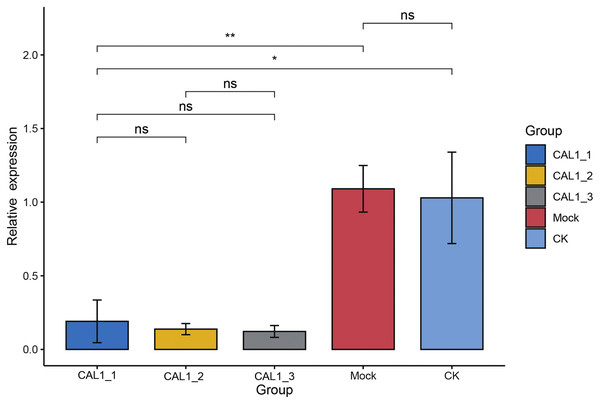
(A) Control plants (CK). (B) Newly formed leaves of H. mutabilis plants infiltrated with pTRV2-HmCLA1 (CLA1) showing white-streaked leaf symptoms after 18 days. (C) Empty vector infiltrated plants (Mock) with the normal phenotype. (D) The leaf phenotypes of the treatments. The leaf on the upper left in Fig. 3D are from plants infiltrated with pTRV2-HmCLA1 (CLA1), the leaf on the upper right is from a control plant (CK) and the bottom one is from an empty vector infiltrated plant (Mock).Subsequently, qPCR was employed to assess the levels of CLA1 mRNA in the infiltrated plants exhibiting white-streaked leaf symptoms. The expression of the CLA1 gene displayed comparable levels between the CK and Mock plants. Conversely, the expression levels of the CLA1 gene were notably reduced by 81.46–92.12% in plants agroinfiltrated with pTRV2-HmCLA1 compared to CK plants (Figs. 4, S2 and Table S2). This expression pattern of HmCLA1 consistently matched the phenotypic features, indicating a notable reduction in HmCLA1 expression via TRV-VIGS in H. mutabilis, ultimately resulting in an albino leaf phenotype.
Figure 4: Relative expression levels of HmCLA1 transcripts in control (CK), empty vector-infected (Mock) and pTRV-HmCLA1-infected plants (pTRV2-HmCLA1).
Error bars indicate the standard errors, and * denotes significant differences at the P < 0.05 level, two asterisks (**) indicate significant differences at the P < 0.01 level, while “ns” indicates no significant difference at the P < 0.05 level.Discussion
Woody plants, encompassing trees, shrubs, and semi-shrubs, serve as essential resources for human sustenance and economic production. However, their extended growth cycles, significant heterozygosity, and limited regenerative capacity present challenges in genetic transformation. The development of cost-effective and efficient techniques, like the VIGS system, is crucial for rapid gene function validation. Presently, only a few vectors have been adopted for VIGS in woody plants. Among the members of the Hibiscus genus, TRV-mediated VIGS systems have been successfully established for H. hamabo and Hibiscus cannabinus (H. cannabinus) (Wang et al., 2019; Yang et al., 2023). Research has also shown that the gene count in the plants of the Hibiscus genus far exceeds that of other plants in the Malvaceae family (Yang et al., 2022). Therefore, the construction of VIGS vectors for other Hibiscus species is of great significance for studying functional genes.
Our study first demonstrated the potential of TRV-VIGS as an effective gene silencing tool in down-regulating endogenous gene expression levels in H. mutabilis. Moreover, the complete genome sequences of H. mutabilis have recently become publicly available (Yang et al., 2022). Knowledge about the complete genome sequence and the availability of TRV-VIGS together could help determine gene functions in H. mutabilis. Thus, the TRV system presented in this study will enhance functional genomic research based on VIGS in H. mutabilis. Further, our research aims to employ the vector system to examine the effects of biotic and abiotic stresses, such as diseases, pests, and low temperatures, on the growth and development of H. mutabilis, to breed more excellent cultivars with strong resistance.
Researches have shown that VIGS outperforms previously developed gene silencing techniques in plants (Beyaz, 2023). Given the current benefits of VIGS technology, numerous VIGS applications in modern biology appear promising. Researchers can utilize the genetic outcomes of VIGS (virus-induced gene silencing) to extensively screen and identify important and astonishing traits in plants (Unver & Budak, 2009). The TRV (Tobacco rattle virus) vector is one of the tools used in VIGS (virus-induced gene silencing) technology, characterized by its ability to overcome host (plant) range and meristem exclusion constraints (Liu, Schiff & Dinesh-Kumar, 2002). The TRV infects a wide range of species, spreads rapidly, and results in relatively mild symptoms in infected plants. Moreover, it can spread within plant meristems, leading to specific target gene degradation. Therefore, it is an ideal vector for analyzing plant gene function (Ratcliff, Martin-Hernandez & Baulcombe, 2001; Shi et al., 2021). Although the range of plants suitable for TRV vectors is relatively wide compared to other VIGS vectors, their efficiency varies from plant to plant and among materials of the same species or genus (Zhang et al., 2016). Brigneti et al. (2004) found that the TRV vector failed to induce effective gene silencing in the potato cultivar Solanum tuberosum cv. Cara. The efficacy with which TRV vector infects woody plants has also been examined. TRV-induced VIGS demonstrated effectiveness in Vernicia fordii, displayed limited potency in Populus tomentosa Carr., and yielded no significant outcomes in Camellia oleifera (Jiang et al., 2014). For Hibiscus, Wang et al. (2019) and Yang et al. (2023) individually applied the TRV-VIGS system to H. hamabo and H. cannabinus, and found that the TRV-VIGS system exhibited remarkable silencing efficiency, resulting in 87% of H. hamabo and 90–100% of H. cannabinus agro-infected plants displaying a white-streaked leaf phenotype. Our study also successfully silenced the CLA1 gene in H. mutabilis cultivar ‘single-petal white’ via VIGS using the TRV system. However, due to the numerous varieties of H. mutabilis, the general applicability of this system requires further research.
CLA1 is a protein that regulates the development of chloroplast and serves as a visual and valuable indicator of the TRV-VIGS system. Multiple sequence alignment of CLA1 from various species in this study revealed that HmCLA1 is highly homologous to the HhCLA1, followed by GhCLA1 protein and GbCLA1 protein. The phylogenetic relationship of the CLA1 protein is generally consistent with that of the family and genus to which it belongs, with one exception being PtCLA1. This discrepancy in PtCLA1’s placement may be attributed to the incomplete sequence data available for PtCLA1.
Conclusions
To conclude, our study demonstrated the efficacy of TRV-mediated VIGS in gene silencing within H. mutabilis, further enriching the roster of woody species amenable to VIGS investigations. The utilization of this loss-of-function assay presents promising prospects for advancing our understanding of the intricate molecular mechanisms governing H. mutabilis growth, development, and response to various stimuli. By silencing specific genes that influence key traits, we can unlock valuable insights into enhancing the species’ overall fitness. For example, we could combine VIGS with next-generation sequencing technology to efficiently analyze and validate the functions of crucial genes in Hibiscus mutabilis in the future.