Research progress on V delta 1+ T cells and their effect on pathogen infection
- Published
- Accepted
- Received
- Academic Editor
- Vladimir Uversky
- Subject Areas
- Cell Biology, Microbiology, Molecular Biology, Immunology, Infectious Diseases
- Keywords
- Vδ1+ T cells, γδT cells, Pathogen infection, Development, Cytokine, Mechanism
- Copyright
- © 2024 Li et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Research progress on V delta 1+ T cells and their effect on pathogen infection. PeerJ 12:e18313 https://doi.org/10.7717/peerj.18313
Abstract
The ongoing high occurrence of harmful infectious diseases significantly threatens human health. Existing methods used to control such diseases primarily involve targeting the pathogens, usually neglecting the vital role of host factors in disease advancement. Gamma delta (γδ) T cells act as a bridge between innate and adaptive immunity, playing a crucial role in combating pathogen invasion. Among these γδT cell subsets, which are categorized based on T cell receptor delta variable expression patterns, V delta (δ) 1+ T cells possess unique recognition abilities and regulatory characteristics and actively engage in various immune responses. The differentiation, development, and immune reactivity of Vδ1+ T cells are closely associated with the initial and progressive stages of infectious diseases. This article provides an overview of the classification, distribution, differentiation, and development of Vδ1+ T cells and their mechanisms in combating pathogenic infections, offering new insights for disease diagnosis and treatment.
Introduction
With the continuous emergence of new and unidentified pathogens and the recurrence of existing pathogens, the incidence of infectious diseases has shown an increasing trend. For example, the spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which caused the rapid infection of millions of people (Senevirathne et al., 2024), and the outbreak of non-epidemic Monkeypox virus in 2022 is regarded as a new global threat (Li et al., 2022; Yi et al., 2024). Other plaguing pathogens, such as Mycobacterium tuberculosis (Mtb), the human immunodeficiency virus (HIV), and Plasmodium falciparum, still have not been completely eradicated. This poses a significant challenge to public healthcare and economic infrastructure. These pathogens enter the body through various means, such as the respiratory, reproductive, and skin pathways. For example, SARS-CoV-2 primarily spreads among people through respiratory secretions, including those from coughing, sneezing, and even droplets produced when talking (Zaidi & Singh, 2024). Therefore, the treatment, prevention, and control of infectious diseases have become the focus and basis of current studies. Similarly, regulating the immune function of the human body to resist pathogen invasion is a recent topic of discussion.
The human immune system responds to microbial infections using various mechanisms to provide protection. As a distinct subclass of T lymphocytes, gamma delta (γδ) T cells serve as a frontline defense in human immune system response. With advancements in studies, emerging evidence has shown that γδT cell functionality extends beyond their innate immune response and is crucial in initiating and orchestrating adaptive immunity. For instance, some γδT cell subsets exhibit professional antigen-presenting cell capabilities (Yang et al., 2022), which are vital for developing and maintaining adaptive immunity and can receive and transmit signals (Perko et al., 2015; Chan et al., 2022). Unlike alpha beta (αβ) T cells, the proportion of γδT cells in the circulating blood is relatively low, accounting for less than 5% of the total T cells in circulation; however, a high proportion of these cells are present in tissues, including the intestinal tissue (approximately 40%) and the skin (10–30%) (Hu et al., 2023). Based on the expression patterns of the T cell receptor delta variable, γδT cells are primarily categorized into three subtypes, namely Vδ1+ T, Vδ2+ T, and Vδ3+ T cells (Zhao et al., 2023). Owing to the limited proportion of Vδ1+ T cells in circulating blood, most studies have primarily focused on Vδ2+ T cells, which are the primary source of γδT cells in circulating blood. However, emerging observations indicate that Vδ1+ T cells also have a crucial impact on disease development (Chan et al., 2024). Therefore, a comprehensive understanding of the traits of Vδ1+ T cells and an in-depth exploration of their role and mechanism in diseases resulting from pathogen infection can provide a clearer study orientation for immunologists, microbiologists, and clinical researchers, providing new insights for disease treatment. Based on this, we wrote this review which focuses on the classification, distribution, differentiation, and development of Vδ1+ T cells and the mechanism underlying their role in infectious diseases.
Survey methodology
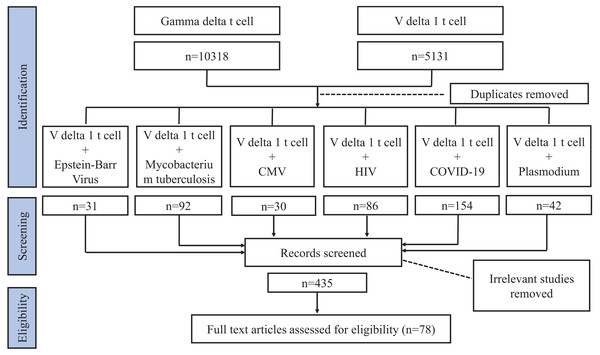
Our review focuses on Vδ1+ T cells and their effect on pathogen infection. We conducted a literature search using the Web of Science and PubMed databases. As Vδ1+ T cells constitute a subgroup of γδT cells, initially, when summarizing the characteristics of Vδ1+ T cells, we used search keywords such as “gamma delta T cell” or “V delta 1 T cell”. Subsequently, when delineating the relationship between Vδ1+ T cell and pathogen infection, in addition to “gamma delta T cell” and “V delta 1 T cell”, the keywords we searched also encompassed “bacteria” or “fungi” or “virus” or “parasite.” Studies related to pathogen infection involving Vδ1+ T cells were screened from these four categories of microorganisms. Further, we searched for “V delta 1 T cell” with “Mycobacterium tuberculosis (MTB)” or “human immunodeficiency virus (HIV)” or “Coronavirus Disease 2019 (COVID-19)” or “Plasmodium” or “cytomegalovirus (CMV)” or “Epstein-Barr Virus (EBV)”. Similarly, some related terms were also considered, such as “TB”, “tuberculosis”, and “SARS-CoV-2”. The articles were checked for compliance with the requirements of this review, excluding irrelevant articles (e.g. changings in Vδ1+ T cells are not caused by pathogenic infections) and articles with older publication years where citations and conclusions were duplicated, and we screened for the results of studies on human specimens and focused on recognized articles with relatively high impact (Fig. 1). We incorporated 78 articles in this review.
Figure 1: Flowchart article selection.
Classification and distribution of Vδ1+ T cells
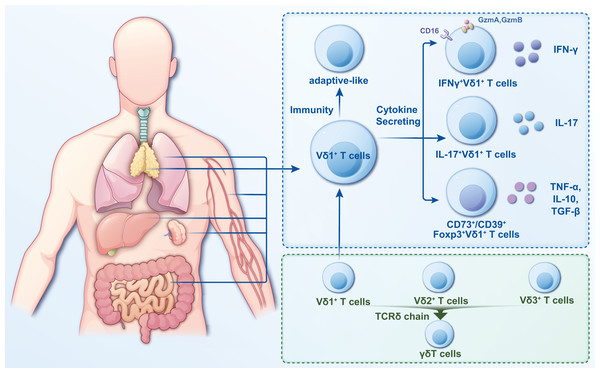
γδT cells are a crucial subgroup of T lymphocytes with distinct characteristics and functions that exhibit features of innate and adaptive immunity (Wang et al., 2024a). Several methods are currently used to classify γδT cells, with the most common being based on the composition of the antigen receptor chains on cell surfaces. The antigen recognition receptor on γδT cells comprises γ and δ chains. Based on their different γ chains, γδT cells can be classified into six different cell subpopulations, namely, Vγ2+, Vγ3+, Vγ4+, Vγ5+, Vγ8+, and Vγ9+; based on their different δ chains, γδT cells are categorized into eight cell subpopulations (Vδ1 through Vδ8) (Dart et al., 2023; Gray et al., 2024). The Vδ2+ subset primarily pairs with Vγ9+ T-cell receptors (TCRs), while the Vδ1+ subset exhibits flexible pairing with various Vγ chains (Hu et al., 2023). Vδ1+ T cells are more distributed (Fig. 2) but in a smaller proportion of peripheral blood and primarily in tissues, including the intestinal epithelium, liver, spleen, and skin (Schadeck et al., 2023).
Figure 2: Classification and distribution characteristics of Vδ1+ T cells.
Vδ1+ T cells are widely distributed and primarily in tissues such as intestinal epithelium, liver, spleen, lung and skin. They are categorized into two distinct classes based on their immunity and cytokine secretion. In the study of infectious diseases, the pro- or anti-infective functions can be more readily described based on the functional or cytokine secretion approach.Human γδT cells, excluding Vγ9Vδ2+ T cells, are categorized as “non-Vγ9Vδ2+ T cells” (Papadopoulou, Sanchez Sanchez & Vermijlen, 2020). A common feature shared by these non-Vγ9Vδ2+ T cells is the adaptive characteristics of their TCR repertoires (Davey et al., 2017). Vδ1+ T cells are an essential part of non-Vγ9Vδ2+ T cells, with diverse and private repertoires (Davey et al., 2017; Gray et al., 2024), including circulating and tissue-resident repertoires (Hunter et al., 2018). In adult intestinal tissues, for example, their private repertoires contain many N additions (Holtmeier et al., 1997). One study evaluated the function of Vδ1+ T cells and Vγ9Vδ2+ T cells at the single-cell level and showed that Vδ1 is more similar to natural killer (NK) cells, whereas Vδ2 is similar to CD8 αβ T cells (Pizzolato et al., 2019). Unlike Vγ9Vδ2+ T cells, Vδ1+ T cells do not recognize phosphorylated antigens; however, they can recognize lipid antigens of the CD1 family, Annexin A2, and MHC class I chain-related protein A and B (Kang, Kim & Lee, 2023; Lien et al., 2024). Furthermore, during pathogenic infections, Vδ1+ T cells increase the expression of natural killer cell receptors and CD16 (FcγRIII). This boosts antibody-dependent cellular cytotoxicity, aiding in recognizing and eliminating pathogens (Field et al., 2024).
Based on cytokine secretion, γδT cells can be grouped into γδT1, γδT2, γδT17, and γδTreg cells, and the associated cytokines are interferon-γ (IFN-γ), interleukin (IL)-4, IL-17A, and transforming growth factor-β, respectively (Das et al., 2024). An increase in the amount of IFN-γ-producing Vδ1 T cells was found in intrahepatic lymphocytes from hepatitis C virus (HCV) infected patients with high liver inflammation which could aggravate hepatic necroinflammation (Agrati et al., 2001). These cells, identified as IFN-γ-producing Vδ1 T cells, exhibit distinct cytotoxic markers (Hu et al., 2023). Moreover, a study that involved using single-cell RNA sequencing to inspect the functional phenotypes of tumor-infiltrating γδT cells in patients with hepatocellular carcinoma revealed that these cells, primarily Vδ1+ T cells, express abundant IFNG and limited IL17A, suggesting that Vδ1+ T cells in tumor tissues exert cytotoxic effects through IFN-γ secretion (Carbone, Vaccher & Gloghini, 2022). Increasing evidence indicates that γδT cells play a regulatory role in cancer or inflammatory diseases (Yao et al., 2022; Das et al., 2024). For instance, in patients with breast cancer, CD73+Vδ1+ T cells constitute most of the tumor-infiltrating cell population and effectively hinder the development and functionality of dendritic cells, similar to the cytokine production by helper CD4+ and effector CD8+ T cells (Wang, Lim & Tan, 2023). These findings suggest that Vδ1+ T cells possess significant functional adaptability, with their function heavily influenced by the surrounding environment.
Differentiation and development of Vδ1+ T cells
The differentiation and development of Vδ1+ T cells are intricate processes governed by numerous factors. The thymus serves as a pivotal organ for T-cell development, where immature T cells progress through various differentiation stages to mature into functional T cells. γδT cells originate from T-cell precursors originating from bone marrow hematopoietic stem cells. In humans, the development of γδT cells requires TCR and sustained Notch signaling, particularly Jagged2/Notch3 signaling (Tubero Euzebio Alves et al., 2024). Differentiation of human γδT cells involves the Notch-independent double-negative (DN) pathway and the Notch-dependent double-positive (DP) pathway. These yield a TCR γδ+ population containing DN, DP, and single-positive (SP) in the postnatal thymus (Van de Walle et al., 2009; Van Coppernolle et al., 2012). This indicates that a fraction of γδT cells express CD8 or CD4 molecules on their surfaces. CD8+γδT cells are primarily formed from Vδ1+ T cells in some pathogen infections (Roy Chowdhury et al., 2023), and their gene transcription levels are highly consistent with those of CD8+αβT cells (McMurray et al., 2022). This suggests that SP γδT cells undergo a developmental process that is similar to that of αβ T cells. Throughout pregnancy, γδT cell subsets shift dynamically, marked by increased Vδ1+ T cell production, which dominates the population in cord blood and the thymus of children, resulting in a higher count of Vδ1+ T cells than Vδ2+ T cells at birth (Boehme, Roels & Taghon, 2022). The frequencies of Vδ1+ T cells and Vδ2+ T cells changed with increasing age. It has been shown that the frequency of Vδ1 cells in the blood decreased significantly at the age of 12, whereas blood Vδ2 cells increased to adult levels; in addition, Vδ1 cells in the lungs increased to adult levels (Gray et al., 2024). The diversity of distribution and tissue specificity of Vδ1+ T cells at different times suggests that they can play critical roles at different times.
The migration of γδT cells from the thymus to peripheral tissues involves various regulatory mechanisms, with the diversity of chemokine receptors on γδT cells playing a crucial role in migration. For example, C-C chemokine receptor six (CCR6) and C-X-C motif chemokine receptor six (CXCR6) on the surface of γδT cells facilitate their migration into mucosal tissues (Moriyama et al., 2023; Das et al., 2024). Vδ1+ T cells, a subgroup of γδT cells, express CCR2 in addition to CCR6 and CXCR6 and show migratory response to C-C motif ligand 2 (Deng et al., 2023). The expression of these chemokine receptors undergoes significant changes in response to different physiological conditions. For example, the inflammatory chemokine receptors CCR2, CCR5, and CCR6 are expressed in the resting state, whereas the lymphatic chemokine receptor CCR7 is activated (Brandes et al., 2003). This indicates that Vδ1+ T cells display tissue-specific homing mediated by chemokine receptors, and in scenarios such as tumor development, infections, and other diseases, Vδ1+ T cells exhibit distinct selective targeting in response to alterations in their microenvironment.
Vδ1+ T cells and pathogen infection
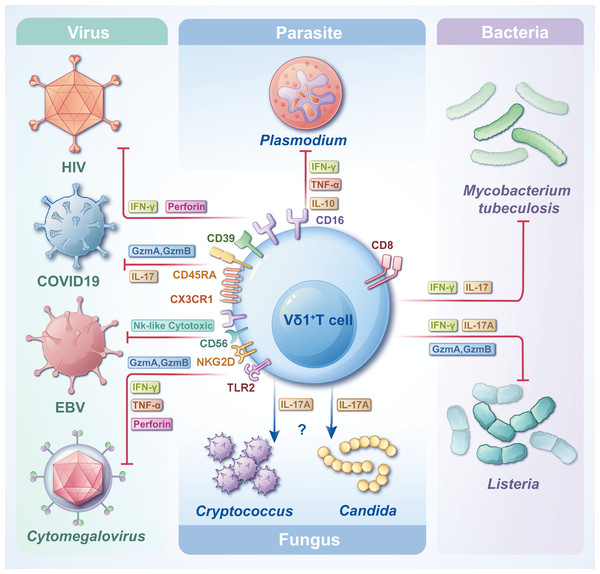
γδT cells are crucial in combating infectious diseases. Upon activation, γδT cells exhibit cytotoxic capabilities, eliminating target cells by releasing cytotoxic substances such as perforin and granzyme, inducing apoptosis. Vδ1+ T cells, functioning as adaptive immune cells, undergo rapid clonal expansion in response to antigenic stimuli from tumors or infectious agents (Chen et al., 2022). Despite their small proportion in the circulating blood, the significance of Vδ1+ T cells in the pathogenesis of infectious diseases is notable. One study analyzed the transcriptional profiles of Vδ1+ T cells and revealed that Vδ1+ Tnaive and Vδ1+ Teffector were highly congruent with CD8+ Tnaive and CD8+ Teffector, respectively, at the cellular, molecular level (including an increase in cytotoxicity and cytokine production) and the gene transcription level (McMurray et al., 2022). Another study on Mycobacterium leprae showed that resident γδT cells in the skin of patients with leprosy can protect against the disease by producing IL-17 in response to IL-23 stimulation, and most of these cells are Vδ1+ T cells. This could provide a potential target for disease treatment (Liu et al., 2022a). The subsequent sections delineate the contributions of Vδ1+ T cells to various types of pathogen infections and their distinct effector functions (Fig. 3). In most cases, Vδ1+T cells can trigger apoptosis in pathogen-infected target cells by secreting perforin and granzymes. Furthermore, they can release various cytokines, including IFN-γ and IL-17, to actively regulate immune responses and facilitate the elimination of pathogens.
Figure 3: The roles of Vδ1+ T cells in infections with various pathogens.
Vδ1+ T cells, when encountering diverse pathogens, express distinct CD molecules and secrete diverse types of cytokines, thereby attaining cytotoxicity and/or immunoregulatory effects. Facing different pathogen infections, Vδ1+ T cells exert different immune mechanisms, which need to be further clarified.Vδ1+ T cells and Plasmodium falciparum infection
Plasmodium falciparum, a prominent malaria parasite affecting humans, imposes significant burdens on social and economic aspects of life. As a distinct subset of T cells, γδT cells are pivotal in combatting Plasmodium infections. Numerous studies have documented a notable and sustained expansion of γδT cells in individuals suffering from Plasmodium infection, revealing an increased abundance and frequency of Vδ1+ T cells (McMurray et al., 2022; Nana et al., 2024). Particularly in malaria-endemic regions, reactive γδT cells in patients, spanning children to adults frequently exposed to P. falciparum, predominantly comprise Vδ1+ T cells. Some studies have suggested that Vδ1+ T cells can help the body to generate naturally acquired immunity against P. falciparum, which is further enhanced when Vδ1+ T cells are induced to mature by the pathogens (Hviid, Smith-Togobo & Willcox, 2019; León-Lara et al., 2022). These cells exhibite a markedly heightened cytotoxic effector phenotype characterized by CD16+CD94negVδ1+ T cells. They demonstrate a versatile cytokine production profile, encompassing IFN-γ, tumor necrosis factor-α, and/or IL-10, with high polyclonality (León-Lara et al., 2022). Notably, recurrent P. falciparum infections trigger pronounced clonal expansion within the Vδ1+ T-cell population, where naïve Vδ1+ T cells gradually transition into cytotoxic Vδ1+ T cells, exerting their cytotoxic effects (von Borstel et al., 2021). These findings could be sufficient to draw our attention to Vδ1+ T cells, and the focus of studies could be shifted from the relationship between γδ T cells and P. falciparum to Vδ1+ T cells with adaptive immune functions.
Vδ1+ T cells and Mtb infection
Tuberculosis (TB), caused by Mtb infection, is a highly contagious airborne disease. According to the World Health Organization’s Global Tuberculosis Report 2023, an estimated 10.6 million individuals worldwide are expected to be infected with TB by 2022. Notably, China alone accounts for approximately 748,000 new TB cases, with approximately 30,000 patients diagnosed with multidrug-resistant TB, and a TB mortality rate of 2.0 per 100,000 individuals (Geneva: World Health Organization, 2023). These data underscore the urgent need to curtail the spread and mutation of Mtb. Previous investigations have revealed that Mtb infection triggers the proliferation of γδT cells in peripheral blood (Roy Chowdhury et al., 2023). Notably, the predominant γδT cell subset in the lung tissue of patients in the acute phase is Vδ1+ T cells (Ogongo et al., 2020), indicating their pivotal role in frontline defense against Mtb infection. Studies have unveiled substantial polymorphism in the γδTCR repertoire using high-throughput immune repertoire sequencing technology (Xia et al., 2023), offering novel insights into the protective mechanisms of γδT cells against Mtb. One study used high-throughput sequencing to screen CDR3δ sequences that can specifically bind to Mtb proteins through the Vδ1 gene sequence. Results demonstrated that stimulation with Mtb proteins triggers significant proliferation and secretion of cytotoxic factors from Vδ1+ T cells in vitro (Li et al., 2019). Additionally, another study revealed a distinct subset of γδT cells, NK-like CD8+ γδT cells, which expand during chronic Mtb infection, primarily comprising Vδ1+ T cells (Roy Chowdhury et al., 2023). Consequently, exploring the mechanism underlying the γδT cell response to Mtb infection is essential, particularly regarding the function of diverse subsets of peripheral blood γδT cells and the functional diversity of these subsets, especially Vδ1+ T cells, at the local infection site.
Vδ1+ T cells and HIV infection
HIV is a retrovirus that specifically targets CD4+ T cells, progressively impairing cellular and adaptive immune responses. Studies have extensively demonstrated the pivotal role of γδT cells in combating HIV infection. A recent study involved using a model of bone marrow-liver-thymus humanized mice to evaluate the quantity and functionality of γδT cells in peripheral blood post-HIV infection (Biradar et al., 2022). These findings are similar to those of previous clinical observations, revealing an increase in Vδ1+ T cells and a decrease in Vδ2+ T cells, altering the Vδ1/Vδ2 ratio, offering a novel perspective for Acquired Immunodeficiency Syndrome. Clinical intervention has increasingly focused on antiretroviral therapy (ART) (Suleman et al., 2024). Studies indicate that post-ART treatment, patients manifest robust activation and degranulation of Vδ1+ T cells, which exhibit a mature NK-like phenotype enriched with CD16 (Pihl et al., 2024; Field et al., 2024). In instances where HIV infection precipitates tumors, Vδ1+ T cells also adopt a cytotoxic phenotype, exerting their cytotoxic effects via IFN-γ secretion (Carbone, Vaccher & Gloghini, 2022). Analysis of HIV-infected patients revealed a notable increase in CD39+Vδ1+ T cells, indicating exhaustion and terminal differentiation, which is independent of the patient’s disease state (Kolbe et al., 2022). Consequently, Vδ1+ T cell character in HIV infection is multifaceted. Although they can identify HIV-infected cells and exert antiviral activity, HIV has evolved mechanisms to evade the immune response, causing impaired Vδ1+ T-cell function. Despite these challenges, comprehending the dynamics of Vδ1+ T-cell responses in the viral infection environment is crucial for devising strategies to bolster their antiviral functions.
Vδ1+ T cells in patients with COVID-19
COVID-19 is caused by SARS-CoV-2. Numerous studies have revealed the effect of COVID-19 on the quantity and functionality of circulating γδT cells (Lin et al., 2024). Investigations have revealed that individuals with acute COVID-19 exhibit a substantial increase in the ratio of effector Vδ1+ T cells (CD27negCD45RA+CX3CR1+) in their circulating blood. Furthermore, the fraction of cytolytic Vδ1+ T cells among GzmA+GzmB+ T cells is markedly elevated, with no such distinction observed for Vδ2+ T cells (von Borstel et al., 2023). Additionally, primate studies underscored a rapidly activating and expanding profile of circulating blood Vδ1+ T cells following SARS-CoV-2 infection. In the initial phases of infection, a correlated relationship was observed between the proportion of Vδ1+ T cells and the viral burden in bronchoalveolar lavage fluid (BALF). Moreover, Vδ1+ T cells in the circulating blood and BALF exhibited a predisposition towards IL-17 secretion, indicating their dual role in viral suppression and proinflammatory responses (Fears et al., 2022). Similarly, as previously mentioned, the prevalence of Vδ1+ T cells was higher in the cord blood and the thymus of children who possessed a naïve Vδ1+ T-cell pool and consequently exhibited a lower susceptibility to COVID-19 infection (Zimmermann & Curtis, 2021). This reveals a potential close association between Vδ1+ T cells and the resilience of children to SARS-CoV-2 infection, underscoring the need for further comprehensive studies on the mechanisms underlying the effect of Vδ1+ T cells on SARS-CoV-2 infection.
Vδ1+ T cells and CMV infection
CMV is an opportunistic β-herpesvirus that is widely distributed and infects most of the world’s population from early childhood (Cannon, Schmid & Hyde, 2010). Studies have shown that the seroprevalence of CMV is approximately 40–90%, and infection in healthy individuals is usually latent and asymptomatic (Seale et al., 2006; Griffiths & Reeves, 2021). However, seropositive individuals have a higher frequency of the Vδ1+ subpopulation than CMV seronegative individuals (Pitard et al., 2008). When the organism is immunocompromised, CMV is reactivated persistently (Chan et al., 2024), a common viral infection complication after allogeneic haematopoietic cell transplantation (Liu et al., 2022b). In hematopoietic stem cell transplantation recipients, CMV reactivation can drive the immune reconstitution of Vδ1+ T cells (de Witte et al., 2018) and the concentration of Vδ1+ T cells was inversely related to CMV reactivation (Bian et al., 2018). Studies have confirmed that Vδ1+γδ T cells are activated and expanded following CMV infection, in transplantation, neonatal infection, or common variable immunodeficiency (Vermijlen et al., 2010; Tuengel et al., 2021; Griffiths & Reeves, 2021). In addition, CMV infection causes a significant incidence of Vδ1+ cells with a terminally differentiated (including high cytotoxic potential) phenotype at the age of 2 years, manifested as CD27−CD45RA+, CD57+, granzyme B+, and CD16+ (Rovito et al., 2017). It has been shown that native and in-vitro-amplified Vδ1+ T cells can directly and independently recognize CMV and CMV-infected cells and inhibit CMV duplication in vitro, which can be achieved through the TCRγδ and toll-like receptor two and natural killer cell group (NKG) 2D receptor pathways (Liu et al., 2022b). The percentage of NKG2C+Vδ1+ T cells is significantly higher, and Granzyme A is strongly expressed in CMV-infected infants and adults compared with that in CMV-uninfected individuals (Tuengel et al., 2021), and Granzyme B is also highly expressed in CMV-infected older adults (Verschoor et al., 2022). The CMV antigens that cause clonal proliferation of Vδ1+ T cells are unknown. Some studies have claimed that Vδ1+ T cells are specific for endothelial protein C receptor (Willcox et al., 2012), and others have revealed that Vδ1+ T cells recognize human leucocyte antigen-DR in a peptide-independent manner (Deseke et al., 2022). These results indicate the significant influence of Vδ1+ T cells in combating CMV infection, and also provide new strategies and directions for the therapy of CMV-infectious diseases.
Discussion
Vδ1+ T cells represent a unique subgroup of γδT cells (10–30% of circulating γδT cells) (Yokobori et al., 2009). Regarding infection, Vδ1+ T cells play vital roles in controlling and eliminating pathogens. Their direct cytotoxicity, cytokine production, and modulation of immune responses significantly contribute to overall immune defense. Their participation in immune responses against infectious diseases underscores their pivotal role in host defense mechanisms. In addition to the aforementioned pathogenic infections, Vδ1+ T cells also undergo clonal expansion and exhibit cytotoxic phenotypes in other infections, including viral infections such as Epstein-Barr virus (EBV) (Hirai et al., 2023), bacterial infections such as Listeria (Wang et al., 2023), and fungal infections such as Candida (Wang et al., 2024b) and Cryptococcus (Sato et al., 2020). These results confirm that the broad pathogen-recognition capacity of Vδ1+ T cells underscores their versatility in combating infections. Moreover, the transcriptional profiles of Vδ1+ T cells are reportedly differentially expressed in various diseases, such as autoimmune diseases (Devan et al., 2024) and malignancy (Ye et al., 2020; Schadeck et al., 2023). Therefore, further investigation into Vδ1+ T cells is required for increased attention. For example, enhancing the quantity or concentration of Vδ1+ T cells with some type of effective expansion protocol or immunostimulant-targeting Vδ1+ T cells, and observing their positive or negative effect on pathogens is essential. This approach will help clarify the direction of the study and disease treatment.
Despite considerable efforts in understanding Vδ1+ T cell biology, numerous questions and challenges persist. Further investigations are required to unravel the factors governing the commitment of thymocytes to the Vδ1 lineage and the mechanisms influencing Vδ1+ T-cell differentiation. Additionally, gaining clearer insights into the interactions between Vδ1+ T cells and diverse pathogens will facilitate the development of targeted therapeutic strategies that can be used to harness the potential of these cells. Some studies have focused on allogeneic Vδ1 CART cell therapy, however, based on the available reports, they have been primarily focused on tumor treatment (Makkouk et al., 2021; Ferry et al., 2022; Sánchez Martínez et al., 2022), while no reports have been published on such therapy for infectious diseases. Therefore, applying the chimeric T cell technique to Vδ1+ T cells may help optimize effectiveness. However, this application is currently making slow progress in the clinic, which may be due to some technical challenges that need further exploration.
Conclusions
The attributes of Vδ1+ T cells in response to pathogen infection, particularly their cytotoxicity and immunomodulatory functions, indicate that they possess significant potential applications in immunotherapy. Vδ1+ T cells are crucial components in the intricate network of immune responses, furnishing an additional layer of defense layer against infectious diseases. Therefore, a deeper comprehension of the development and biological function of Vδ1+ T cells is anticipated to facilitate the understanding of immunity and foster innovative approaches for preventing and treating infectious diseases. The investigation of Vδ1+ T cells is an ongoing process, and their distinctive characteristics are expected to be leveraged to develop Vδ1+ T cell therapy for the treatment of clinical diseases. The further exploration of Vδ1+ T cells is expected to offer more possibilities for the future development of cellular immunotherapy.