Klastomycter conodentatus, gen et sp. nov., a small early Permian parareptile with conical teeth from Richards Spur, Oklahoma
- Published
- Accepted
- Received
- Academic Editor
- Joseph Gillespie
- Subject Areas
- Evolutionary Studies, Paleontology
- Keywords
- Paleontology, Parareptilia, Crania, Dentition, Parareptile diversity, Acleistorhinidae
- Copyright
- © 2024 Reisz et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Klastomycter conodentatus, gen et sp. nov., a small early Permian parareptile with conical teeth from Richards Spur, Oklahoma. PeerJ 12:e18393 https://doi.org/10.7717/peerj.18393
Abstract
A small, pristinely preserved specimen recently collected from the Dolese Brothers limestone quarry near Richards Spur, Oklahoma provides evidence for the presence of a new early Permian parareptile at this locality. The specimen includes an articulated, nearly complete skull roof, and with the right premaxilla, right quadratojugal, most of the right palate, as well as the right epipterygoid and the sphenethmoid preserved inside. Although similar in many respects to the other contemporary parareptiles Acleistorhinus, Delorhynchus and Colobomycter, it can be distinguished from other acleistorhinids by the presence of a number of autapomorphies related to its dentition. Phylogenetic analysis places it closer to Delorhynchus and Colobomycter within Acleistorhinidae than to Acleistorhinus pteroticus. Unique aspects of the present specimen include the pronounced anterior extension of the lacrimal bone, largely homodont dentition composed of simple conical crowns with slight recurvature in the premaxillary and anterior maxillary teeth, and simple conical crowns in posterior maxillary dentition. The discovery of this new parareptile along with the surprisingly large number of acleistorhinids at Richards Spur highlights the importance of the unique fissure and vertical cave system at this site. No other early Permian site has provided such a wide diversity of parareptilian taxa, part of a complex community of terrestrial vertebrates. The present specimen highlights the fine niche partitioning that appears to have been present among reptiles of this region.
Introduction
The Dolese Brothers Limestone Quarry near Richards Spur, Oklahoma preserves a complex cave system that has yielded since the early 20th century a vast number of terrestrial tetrapod fossils dating back to the early Permian (289 Ma.) (MacDougall et al., 2017a). Over 30 taxa have been identified at this locality, making it the most taxonomically rich site for Paleozoic terrestrial tetrapods yet discovered (Sullivan, Reisz & May, 2000; MacDougall et al., 2017a; MacDougall et al., 2017b). The fossil preservation observed at Richards Spur is, in part, a result of large crevices in the rock which were open to the ground surface during the Lower Permian (Olson, 1991). This vertical cave system was likely detrimental to many of the terrestrial tetrapods of the time, as the crevices could reach a depth of more than 30 metres (Olson, 1991; Sullivan, Reisz & May, 2000). Remains of animals that either suffered a fatal accident, inhabited the areas around the openings of the crevices and were washed in by monsoonal rainfall or somehow ended up in the crevices by other means became preserved through geological time as clay and other Permian sediments filled the caves (Sullivan, Reisz & May, 2000; MacDougall et al., 2017a; MacDougall et al., 2017b). This natural trap has allowed for the preservation of small Permian tetrapods in a way which has not been seen anywhere else. What were once large crevices often acting as natural traps for terrestrial vertebrates are now exposed to us as fissures at Richards Spur through excavations for the surrounding Ordovician limestone where the caves first developed (Sullivan, Reisz & May, 2000; MacDougall et al., 2017a; MacDougall et al., 2017b).
Among the many terrestrial tetrapods found at Richards Spur are those belonging to Parareptilia, a group of reptiles that were relatively rare during the early Permian but became very common towards the end of that period (MacDougall et al., 2019). The importance of Richards Spur cannot be overstated as it has provided us with most of the early Permian parareptiles that represent the initial stages of diversification of this clade. Genera found at Richards Spur include Colobomycter (two species), Delorhynchus (three species), Feeserpeton, Bolosaurus, Microleter and Abyssomedon (Vaughn, 1958; Fox, 1962; Daly, 1969; Reisz, Barkas & Scott, 2002; Tsuji, Müller & Reisz, 2010; MacDougall & Reisz, 2012; Rowe et al., 2021). The discovery of this small partial skull, which is described here, adds another genus to this list. This fossil specimen includes an articulated skull roof and several palatal elements. The superficial similarity of this specimen to that of Acleistorhinus pteroticus, a parareptile found at another locality in Oklahoma, provides support for the idea that this animal is an acleistorhinid (DeBraga & Reisz, 1996). While these two specimens closely resemble one another at first glance, several key features can be identified which support the identification of this parareptile as a new genus separate from that of Acleistorhinus pteroticus.
Material and Methods
Non-destructive, thermal-neutron microtomographic measurement of specimen BMRP2008.3.3 was performed using the DINGO thermal neutron radiography/tomography/imaging station, located at the 20 MW Open-Pool Australian Lightwater (OPAL) reactor housed at the ANSTO, Lucas Heights, New South Wales, Australia. Data was collected following the methodology of Rowe et al. (2021), with the exception of a pixel size of 14.5 × 14.5 µm and a field of view of 43 × 73 mm2 during the operation of DINGO. In addition, the tomographic scan consisted of 1,000 shadow radiographs obtained every 0.18°, and the exposure length was 15 s (Mays, Bevitt & Stilwell, 2017) with a total scan time of 18 h. Neutron activation was measured at 1 h upon completion of scan, at 3 days and one week in contrast to 35 min, 4 days and 2 weeks as in Rowe et al. (2021).
Once the scanned specimen was available, the obtained images were first refined using ImageJ and then imported into Avizo Lite for segmentation. Figures were then assembled in Adobe Photoshop Elements 8.0 and Adobe Illustrator. The phylogenetic analysis conducted in this study follows the methodology of Rowe, Bevitt & Reisz (2023), with the analysis performed in PAUP 4.0a169 (heuristic search with 1,000 replicates performed via stepwise addition) and the matrix updated in Mesquite.
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:20C50EBB-182E-4A9D-9559-4F518034AFBB. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central SCIE and CLOCKSS.
Systematic Paleontology
| Clade Parareptilia Olson, 1947 |
| Clade Ankyramorpha DeBraga & Rieppel, 1996 |
| Family Acleistorhinidae (Daly, 1969) |
| Genus Klastomycter gen. nov. |
| Specific epithet conodentatus sp. nov. |
Diagnostic Features: Parareptile characterized by the following apomorphies: presence of conical homodont dentition which is slightly recurved apically, and presence of a sphenethmoid with pronounced medial curvature of the dorsal processes. Can be distinguished from Colobomycter by the presence of a shallowly concave lateral skull margin and four premaxillary teeth, a nearly straight nasal-frontal suture, a wide contribution of the postorbital to the temporal fenestra. Differs from Delorhynchus by the fewer number of maxillary teeth (19 maxillary teeth versus 24), a triradiate jugal; absence of tuberosities on the dorsal skull roof excluding the orbital region, absence of an anterolateral palatine process, and an open orbitonasal foramen. Can be distinguished from Acleistorhinus pteroticus by the presence of a straight posterior orbital margin, a pointed anterior process of the quadratojugal, an enlarged vomerine tooth, a tooth field extending to the lateral margin of the palatine, and an extension of the palatal process of the pterygoid reaching to the middle of the vomer.
Holotype: BMRP2008.3.3, a partial skull excluding mandible.
Locality and Horizon: Dolese Brothers Limestone Quarry, Richards Spur, Comanche County, Oklahoma. Absolute dating indicates an age of 289-286 Ma corresponding to the Sakmarian stage of the early Permian (Woodhead et al., 2010; MacDougall et al., 2017a; MacDougall et al., 2017b).
Etymology: Genus name Klastomycter (klasto, Gr., broken, and mycter, Gr., nostril) refers to the disarticulated premaxilla. Specific epithet conodentatus (cono, Gr., conical, and dentatus, Gr., tooth) was chosen on the basis of the conical shape of teeth within this acleistorhinid parareptile.
Description
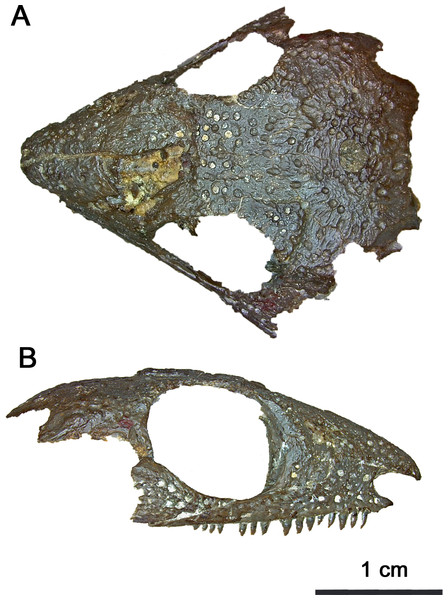
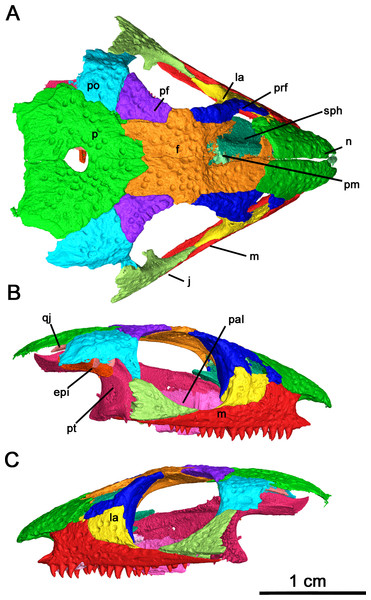
General cranial and postcranial proportions: The skull of the holotype and only known specimen, BMRP2008.3.3, is approximately 29.4 mm in length, with an orbital length of 9.0 mm resulting in a ratio of skull length to orbital length of 3.25 (Figs. 1–3). Wide, shallow pits are scattered on the lateral surfaces of all preserved elements of the skull roof excluding the quadratojugal. In comparison to other acleistorhinids, Klastomycter lacks pronounced tuberosities on the dorsal skull roof which matches the original description of Acleistorhinus (Daly, 1969).
Figure 1: Photographed holotype of Klastomycter conodentatus.
(A) Dorsal and (B) right lateral views.Figure 2: Holotype of Klastomycter conodentatus.
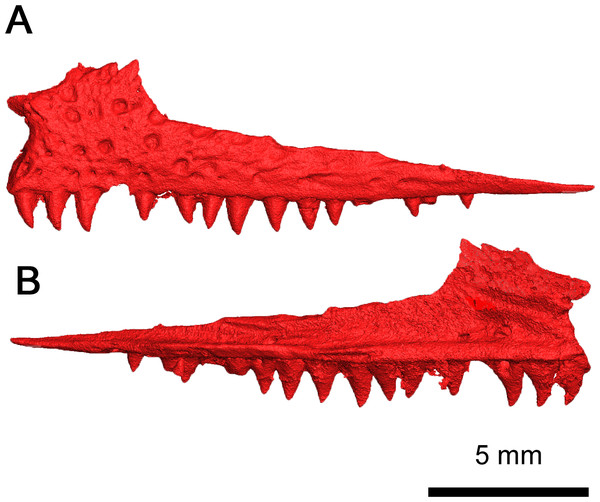
(A) Dorsal, (B) right lateral and (C) left lateral views. Abbreviations: epi, epipterygoid; f, frontal; j, jugal; la, lacrimal; m, maxilla; n, nasal; p, parietal; pal, palatine; pf, postfrontal; pm, premaxilla; po, postorbital; prf, prefrontal; pt, pterygoid; qj, quadratojugal; sph, sphenethmoid.Figure 3: Klastomycter conodentatus in ventral view.
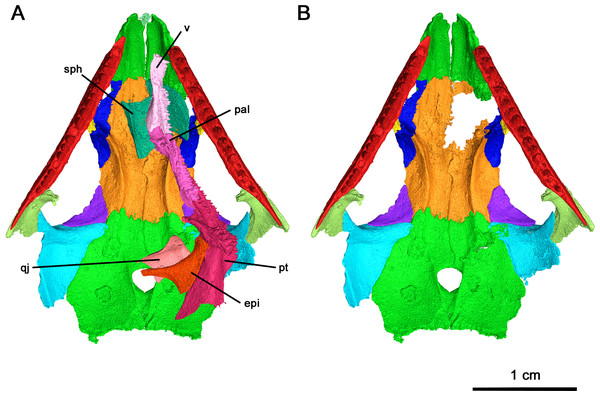
(A) Full specimen in ventral view and (B) ventral skull roof. Abbreviations: epi, epipterygoid; pal, palatine; pt, pterygoid; qj, quadratojugal; sph, sphenethmoid; v, vomer.Skull Roof: In this specimen the right premaxilla is completely preserved, albeit disarticulated, whereas the right premaxilla is only present as a thin fragment of the dorsal process articulating with the nasal (Fig. 4). The premaxilla is slender rather than broad, with the angle between the midline of the premaxilla and the lateral edge being smaller than the condition described in Acleistorhinus pteroticus and Colobomycter pholeter (DeBraga & Reisz, 1996; MacDougall et al., 2017b), indicative of an unusually narrow snout. Anteriorly, the rostral end of the premaxilla is rounded in a similar fashion to that of A. pteroticus rather than the pointed condition of C. pholeter (Daly, 1969; MacDougall et al., 2017b). The premaxilla has a thin, curved dorsal process which would connect to the nasal to form the medial border of the external nares. Compared to A. pteroticus, the dorsal process is slender and more closely resembles the condition of C. pholeter. Anteriorly, the base of the dorsal process possesses a small indentation, which may have been a foramen for a premaxillary nerve canal. The tip of the dorsal process has a small groove on its lateral surface, which would have most likely connected to the nasal. Dorsally and ventrally, the premaxilla is V-shaped with a palatal process projecting posteromedially that is half the length of the premaxillary contribution to the alveolar margin. This palatal process is fairly robust, with a bifurcated tip that would have most likely contacted the anterior vomer. The alveolar margin possesses the same sutural surfaces for the maxilla and septomaxilla as C. pholeter. There are four tooth positions on the right premaxilla of this species, as in Acleistorhinus. Unfortunately, the tooth belonging to the first position is missing, but by the size of the remaining cavity it can be inferred that it was approximately the same size as the second tooth which is approximately 1.38 mm tall. In contrast, the third and fourth premaxillary teeth are 1.35 and 0.85 mm tall respectively, resulting in a gradual decrease in size of the premaxillary tooth positions. The alveolus of the first premaxillary tooth position suggests that the premaxillary teeth and maxillary teeth are approximately equal in length at their longest, as the largest maxillary tooth is 1.37 mm in crown length. The mostly uniform length of premaxillary and maxillary teeth differs from what is seen in A. pteroticus, in which the largest tooth present is present on the maxilla (DeBraga & Reisz, 1996). Each of the three premaxillary teeth which have been preserved in this specimen are conical and compressed in shape with modest recurvature. The shape and number of premaxillary teeth are poorly known in acleistorhinids, only the holotype of Acleistorhinus has this part of the snout preserved.
Figure 4: Left premaxilla of Klastomycter conodentatus.
(A) Medial view, (B) ventral view and (C) lateral view.Figure 5: Left maxilla of Klastomycter conodentatus.
(A) Lateral and (B) medial views.Both maxillae are present, and they remain in proximity with almost all the surrounding skull roof elements (Figs. 2B, 2C and 5). This dentigerous element is mostly complete on both sides, with the exception of the left premaxillary process. The overall morphology of the maxilla closely resembles Delorhynchus cifellii (Reisz, Macdougall & Modesto, 2014), although the anterior portion of the dorsal process connects directly to the nasal instead of the anteriormost lacrimal. The anterior part of the maxilla possesses several supralabial foramina, the largest of which is the anterolateral maxillary foramen (Reisz, Macdougall & Modesto, 2014). This increased size of the anterolateral maxillary foramen, compared to other maxillary foramina, is a shared trait among parareptiles (DeBraga & Reisz, 1996). The preserved premaxillary process in this specimen closely resembles the morphology of Delorhynchus, whereas in Colobomycter pholeter and Acleistorhinus pteroticus it is more robust. As with other acleistorhinids, the maxillary portion of the external nares is bordered ventrally by the premaxillary process and posteriorly by the dorsal lamina, which projects anterodorsally as described in C. pholeter and D. cifellii (MacDougall et al., 2017a; MacDougall et al., 2017b; Rowe, Bevitt & Reisz, 2023). The dorsal lamina of the maxilla in the current specimen is semirectangular, whereas in Acleistorhinus the dorsal process is rounded (DeBraga & Reisz, 1996). While not as prominent as in Delorhynchus, an anterodorsal projection of the dorsal lamina contributes to the posterodorsal part of the external nares. This specimen has 19 tooth positions on each maxilla. The overall number of tooth positions therefore resembles Acleistorhinus most closely, which possesses seventeen teeth on each maxillary element (DeBraga & Reisz, 1996). In contrast, D. cifellii possesses twenty-four maxillary tooth positions, whereas C. pholeter has only thirteen tooth positions due to its enlarged caniniform teeth (Reisz, Macdougall & Modesto, 2014; MacDougall et al., 2017a; MacDougall et al., 2017b). Posteriorly, the maxilla contributes to the ventral margin of the orbits, and extend past their posterior margin terminating in a thin triangular process. Based on morphology of the jugal and the quadratojugal, the posterior end of the maxilla would form a point contact with the quadratojugal. In contrast to what is seen in Acleistorhinus, the tooth bearing region of the maxilla does not extend past the posterior orbital margin (DeBraga & Reisz, 1996).
The marginal dentition in BMRP2008.3.3 differs from the relatively larger and heterodont dentitions in Colobomycter pholeter and Acleistorhinus pteroticus in that the teeth are conspicuously smaller and generally homodont. As in Delorhynchus cifellii, this species does not have pronounced caniniform teeth. The premaxillary teeth are the largest of the marginal dentition, followed closely by the maxillary teeth occupying positions 1-4. After this point, the teeth slightly shrink in size with slightly larger teeth reappearing to occupy positions 7-10. The teeth are conical and compressed with only a very slight amount of recurvature. The tips of the teeth form a sharp point as opposed to the more rounded condition seen in D. cifellii and A. pteroticus. This dentition is distinctive in that even the largest teeth are conical in shape rather than columnar. The teeth on this specimen are most reminiscent to that of D. cifellii because of the generally homodont dentition, but the conical shape of the teeth seen in this species is unique and differs from that of other acleistorhinids, including D. cifellii, A. pteroticus and C. pholeter. BMRP2008.3.3 displays a pleuroacrodont method of dental implantation, which has been previously observed in D. cifellii (Haridy, MacDougall & Reisz, 2018). In addition, resorption pits are present on teeth 3 and 11 of the left maxilla in Fig. 3, and a replacement tooth between tooth positions 14 and 15 indicates a labio-vertical method of tooth replacement. Plicidentine is also present at the base of the teeth in BMRP2008.3.3, as with D. cifellii (MacDougall, LeBlanc & Reisz, 2014)
The anterior portion of the nasal forms the dorsal margin of the external nares and contacts the anterodorsal process of the maxilla by its ventrolateral edge (Figs. 2A and 3B). It is connected dorsolaterally to the prefrontal, dorsally to the frontal and ventrolaterally to the anterior process of the lacrimal. The outline of the nasal forms a quadrangular shape and, as is seen in Acleistorhinus pteroticus, the nasal of this specimen is wider posteriorly rather than anteriorly. However, similarly to A. pteroticus, the nasal of this species forms a nearly straight suture with the frontal, rather than a jagged suture as is seen in Colobomycter pholeter and Delorhynchus cifellii.
The lacrimal has a long anterior process which overlaps the medial surface of the dorsal process of the maxilla and meets the ventrolateral edge of the nasal (Figs. 2B and 2C). This anterior process is partially hidden in lateral view, in contrast to the more exposed anterior process seen in Delorhynchus cifellii. In addition, the anterior process terminates at the posterolateral corner of the nasal, instead of the partial separation found in D. cifellii (Reisz, Macdougall & Modesto, 2014). The lateral surface of the lacrimal is bordered ventrally by the maxilla, anteriorly by the posterodorsal process of the maxilla and dorsally by the prefrontal and the nasal. The posterolateral exposure of the lacrimal is semilunar in shape and makes up the anteroventral corner of the orbit (Fig. 2C), which is bordered dorsally and medially by the prefrontal. Posteroventrally, the lacrimal also has an elongated, thin process which overlaps the medial surface of the maxilla and is not visible in lateral view. This thin process ends in a point contact with the anterior process of the jugal much like what is seen in D. cifellii and Colobomycter pholeter. In contrast, A. pteroticus has a more substantial contact between the lacrimal and the jugal (DeBraga & Reisz, 1996). There are two foramina on the posterior surface of each lacrimal, with the more dorsal foramen being larger than the ventral foramen. These foramina differ from what is seen in D. cifellii, where the ventral foramen is larger than the dorsal foramen.
Although neither prefrontal is fully preserved, a complete picture of this bone is provided by both elements (Fig. 2). The dorsal face of the prefrontal connects medially to the frontal, anteromedially to the nasal, anterolaterally to the posterior of the dorsal process of the maxilla and laterally to the lacrimal. Anteriorly, the dorsal surface of the prefrontal is thin and tapered like in Delorhynchus cifellii, unlike the wide anterior prefrontal seen in Acleistorhinus pteroticus (DeBraga & Reisz, 1996; Reisz, Macdougall & Modesto, 2014). The posterior surface of the prefrontal makes up the anterior orbit and the anterior first third of the dorsal orbit. Much of this posterior surface sits along the medial surface of the dorsal lacrimal, and these two elements makes up the entire anterior orbit. A thin antorbital wall projects from the ventral surface of the prefrontal, suturing onto the lacrimal as in Colobomycter pholeter and C. vaughni with the contribution of the prefrontal resembling the latter more closely (MacDougall, Modesto & Reisz, 2016; MacDougall et al., 2017a; MacDougall et al., 2017b).
Most of the frontal is preserved in Klastomycter conodentatus, with only the anterior portion of the left frontal missing. This element comprises a large portion of the skull roof, connecting anteriorly with the nasal, anterolaterally with the prefrontal, posterolaterally with the postfrontal and posteriorly with the parietal (Fig. 2A). The frontal possesses a lateral lappet which makes up approximately one third of the dorsal orbit and separates the prefrontal and postfrontal bones. Compared to Acleistorhinus pteroticus, the frontal of this species extends farther posteriorly and is more alike to the condition seen in Delorhynchus cifellii and Colobomycter pholeter (DeBraga & Reisz, 1996). Additionally, as with these two taxa, the frontal of the current specimen forms a slanted, somewhat jagged connection to the parietal. This is different from the straighter yet gently rounded connection seen in A. pteroticus. Ventrally, the antorbital ridge continues onto the frontal, decreasing in height posteriorly as in D. cifellii (Rowe, Bevitt & Reisz, 2023).
The postfrontal has a triangular outline in dorsal view and is connected medially to the frontal, posteriorly to the parietal and posterolaterally to the postorbital (Fig. 2A). Anterolaterally, the slightly curved edge of the postfrontal comprises the posterior third of the dorsal orbit. The postfrontal of the current specimen does not extend posteriorly, and rather than wedging between the parietal and the postorbital as in Acleistorhinus pteroticus it terminates just before the connection between these two elements (DeBraga & Reisz, 1996). This is reminiscent of what is seen in Colobomycter pholeter and Delorhynchus cifellii but with less jagged sutures than that of the latter.
The jugal is a triradiate element connected anteriorly to the lacrimal, ventrally to the maxilla, dorsally to the postorbital and likely posteroventrally to the quadratojugal (Figs. 2B and 2C). The anterodorsal edge of the jugal forms the posterior two thirds of the ventral margin of the orbit and part of the posterior margin of the orbit. Much like Acleistorhinus pteroticus and Colobomycter pholeter, the triradiate shape of the preserved jugal confirms that this parareptile had a single lateral temporal fenestra on either side of the skull. However, the reduced dorsal process of the jugal as compared to that of A. pteroticus suggests that the lateral temporal fenestrae were bordered by the jugal, the postorbital, the squamosal and the quadratojugal, as is seen in Delorhynchus cifellii and C. pholeter. If the dorsal process was taller, as in A. pteroticus, the postorbital would not be included in the border of the lateral temporal fenestrae. More support for this idea comes from the fact that the jugal of this specimen is most similar to that of C. pholeter, where a single lateral temporal fenestra is bordered by these four elements. Unfortunately, because the squamosal was not preserved in this specimen, its contribution to the temporal fenestra is not certain. Medially, the jugal has a short ramus which projects towards the midline of the skull, as in Delorhynchus cifellii (Rowe, Bevitt & Reisz, 2023).
Some damage has occurred to each side of the postorbital but their contribution to the ventral and posterior orbit is still evident (Fig. 2). The postorbital is connected ventrally to the jugal, dorsally to the parietal and anterodorsally to the prefrontal. If the squamosal was preserved, the postorbital would likely be connected to it by the posterior end of its ventral edge, as is seen in Colobomycter pholeter and Delorhynchus cifellii. This differs significantly from Acleistorhinus pteroticus, in which the postorbital does not contact the parietal as it is separated from this element by the postfrontal and supratemporal. Additionally, the similarity of the postorbital morphology to C. pholeter suggests that it contributed to the border of the lateral temporal fenestrae, and the squamosal likely did not wrap around the ventral edge of the postorbital to connect to the jugal. This would prevent this element from contributing to the lateral temporal fenestra as is seen in Acleistorhinus pteroticus.
The parietal is a broad, flat element making up the posterior border of the skull roof. In dorsal view, the anterior portion of the parietal forms a rounded point where it meets with the frontal (Fig. 2A). The parietal connects anterolaterally to the prefrontal and laterally to the postorbital, and potentially the supratemporal if it was preserved. The groove on the parietal where the supratemporal would have fit can be clearly seen on the posterolateral portion of the element, and suggests that the bone was large, as in other acleistorhinids. In this species, the pineal foramen is in the middle of the two parietal elements. This can be contrasted to Acleistorhinus pteroticus, in which the pineal foramen is displaced anteriorly, closer to the frontoparietal suture. The condition displayed here is common to both Colobomycter pholeter and Delorhynchus cifellii and is believed to be the primitive condition of the trait (DeBraga & Reisz, 1996). The shallow dimpling present on all skull roof elements is concentrated around the pineal foramen.
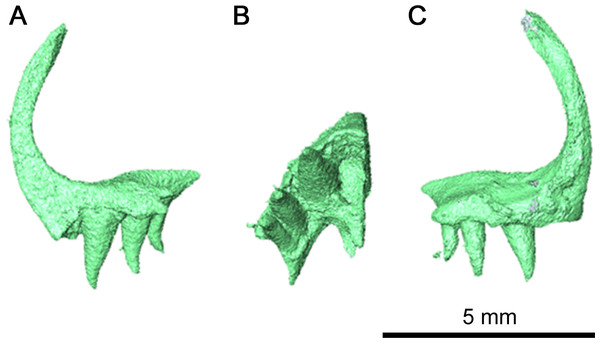
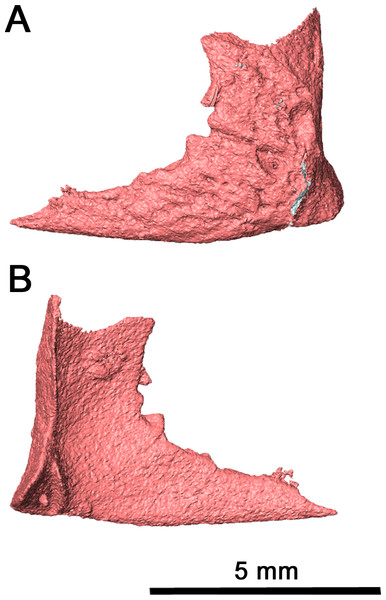
While the left quadratojugal is disarticulated, it is still present and complete (Fig. 6). It is likely that this posterior skull roof element contacted the jugal and the maxilla anteriorly and the squamosal dorsally. This placement means that the quadratojugal contributed to the ventral and posteroventral margin of the lateral temporal fenestra. The shape of the quadratojugal in this species is very similar to that of Acleistorhinus pteroticus, with a concave dorsal edge differing from the condition seen in Colobomycter pholeter where the dorsal edge of the quadratojugal forms a rounded edge. This distinction is important as it indicates that the squamosal in this species likely did not have a ventral process curving around the quadratojugal. Instead, the squamosal likely had a slightly rounded but overall flat dorsal edge which connected with the quadratojugal, as is seen in Acleistorhinus pteroticus. Laterally, the quadratojugal is rugose as in C. pholeter and Delorhynchus cifellii (MacDougall, LeBlanc & Reisz, 2014; MacDougall et al., 2017a; MacDougall et al., 2017b), while lacking the shallow pits seen elsewhere on the skull roof.
Figure 6: Left quadratojugal of Klastomycter conodentatus.
(A) Lateral view and (B) medial view.Palate & braincase
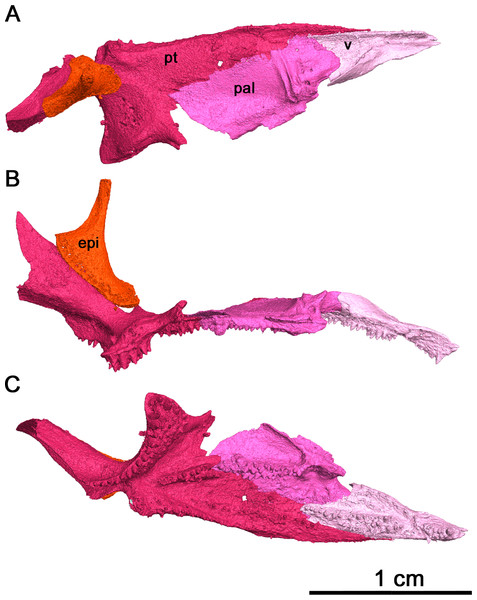
Of the dentigerous elements noted in other acleistorhinids, only the vomer, palatine and pterygoid have been preserved in this specimen with a significant portion of the ventral surface covered by tooth fields (Fig. 7). Ventrolaterally, the sulcus extends through the palatine and the pterygoid, and would most likely extend through the ectopterygoid as in Delorhynchus cifellii (Rowe, Bevitt & Reisz, 2023). However, the channel of the choana is separated from the dentigerous portion of the palatine by a thin ridge.
Figure 7: Right palate of Klastomycter conodentatus.
(A) Dorsal view, (B) lateral view and (C) ventral view. Abbreviations: epi, epipterygoid; pal, palatine; pt, pterygoid; v, vomer.In palatal view, the vomer is an elongate triangular element (Fig. 7). Posteriorly, the vomer increases in width before narrowing as it wedges between the palatine and pterygoid. The vomer is connected by the posterior portion of its medial edge to the pterygoid and by its posterolateral edge to the palatine. If the paired vomer elements were present, the two would likely meet anteriorly along their medial edge, forming the anterior midline of the palate. The posterior medial edge of the vomer would be separated from its pair by the anterior tip of the pterygoid. The anterior vomer is angled ventrally, as can be seen in lateral view. More than half of the ventral surface of the vomer is covered in teeth. The largest tooth occupies the anteriormost tip of the vomer, a condition shared by Delorhynchus cifellii and Colobomycter pholeter, and is the largest tooth on the palatal surface. Behind the enlarged tooth is a field of teeth located medially which spans the length of this element. This tooth field extends onto the pterygoid and is three teeth wide at its widest extent. The vomer of the current specimen shows similarities to that of C. pholeter, Acleistorhinus pteroticus and D. cifellii in terms of its triangular shape. However, the tooth fields in this specimen closely resemble D. cifellii, and are distinct from C. pholeter and A. pteroticus (DeBraga & Reisz, 1996; Modesto & Reisz, 2008; Rowe, Bevitt & Reisz, 2023). On its dorsal surface, the vomer possesses an alar flange extending posterolaterally onto the palatine towards the anterior edge of the posterior external nares. The vomer is slightly disarticulated here, as it would connect to the orbitonasal ridge of the palatine and form the medial wall of the choana as in D. cifellii. However, the alar flange is comparatively more delicate in Klastomycter conodentatus.
The palatine is a quadrangular element which connects anteriorly to the vomer and medially to the pterygoid (Fig. 7). In full articulation, it would also connect laterally to the maxilla and dorsally to the lacrimal and the prefrontal. One large tooth field occupies the palatine, starting approximately one third from the anteriormost end at the mediolateral midline of the element and continuing posteromedially across it. This diagonal tooth field then continues across the posteromedial corner of the palatine on to the pterygoid. The anterior end of this tooth field starts as what appears to be a single tooth row, widening to accommodate up to three rows before reducing to two rows on the pterygoid. This differs from Acleistorhinus pteroticus in that this tooth field continues farther anteroventrally onto the palatine in Klastomycter conodentatus, and the tooth field accommodates three rows of teeth rather than two. Compared to Delorhynchus cifellii, the palatine of K. conodentatus has a smaller concentration of teeth. In contrast to the condition in K. conodentatus, in Colobomycter pholeter, the pterygopalatal tooth field is focused more on the palatine rather than on the pterygoid, and becomes larger as it extends onto the pterygoid. The dorsal surface of the palatine possesses an orbitonasal ridge extending laterally towards the posterior edge of the posterior external nares. Comparisons to A. pteroticus and C. pholeter are not currently possible, but D. cifellii possesses a similar set of dorsal ridges. The orbitonasal ridge is open dorsally, unlike D. cifellii, where the orbitonasal ridge is enclosed. In addition, unlike the condition in D. cifellii and C. pholeter, there is no anterolateral process of the palatine that would border the choana laterally (MacDougall et al., 2017a; MacDougall et al., 2017b; Rowe, Bevitt & Reisz, 2023).
As with many other early Permian amniotes, the pterygoid is a large triangular element composed of an elongated palatal process, a wide transverse process and a posterior quadrate ramus (Figs. 7A and 7C). The palatal process of the pterygoid borders the posteromedial edge of the vomer anteriorly and terminates in a sharp point, and contacts the palatine laterally. There are three distinct tooth fields on the ventral surface of the palatal process, with the first tooth field originating at the posteromedial corner of the palatal process and continuing anteriorly along the medial border of the pterygoid. This tooth field becomes smaller in both the number of tooth rows and the size of individual teeth until it disappears entirely about halfway along the medial edge of the palatal process. The second tooth field on the palatal process of the pterygoid continues onto the pterygoid from the vomer, located along the longitudinal axis of the palatal process of the pterygoid and disappearing about a third of the way from the posterior edge of this element. The last tooth field located on the ventral surface of the pterygoid is a continuation of the tooth field of the palatine and is composed of two rows of teeth that extend diagonally across the pterygoid before terminating just before the quadrate ramus. The locations of the tooth fields on the palatal process of the pterygoid are similar to that seen in Acleistorhinus pteroticus but the fields themselves are much larger, consisting of more rows of teeth and larger teeth. Overall, the tooth fields on the current specimen take up a larger proportion of the ventral surface area of the pterygoid than in A. pteroticus. This condition also differs from that seen in Colobomycter pholeter and Delorhynchus cifellii as the teeth occupying this region in these species seem to be more numerous yet more spread out.
The transverse process of the pterygoid extends ventroposterolaterally from the posterolateral edge of the palatal process of the pterygoid. In ventral view the transverse process is triangular in shape, and nearly the entirety of the ventral surface of this process is covered in teeth. These teeth make up the fourth tooth field present on the pterygoid of this species, bordering the posterior edge of the transverse process and continuing medially onto the raised ventral edge of the quadrate ramus. Laterally, this tooth row wraps around the posterolateral corner of the ventral face of the transverse process. The teeth located on this lateral edge are some of the largest present on the palate, with four teeth on the posterior transverse process that are nearly as large as the single large tooth occupying the anteriormost tip of the vomer. In addition to the distinct row of teeth on the transverse process, there is a large cluster of smaller teeth which cover the rest of the ventral surface of this process. At its widest point, the tooth field on the transverse process is made up of five rows of teeth. This tooth field is much larger and wider than that seen in Acleistorhinus pteroticus, in addition to the fact that the teeth themselves making up this field are much larger in Klastomycter conodentatus. The transverse process of the pterygoid is more similar to that seen in Colobomycter pholeter and Delorhynchus cifellii.
Posteriorly, the quadrate ramus of the pterygoid is a large semiconical sheet of bone which extends posterodorsally from the posterior edge of the dorsal surface of the palatine process (Fig. 7). As it extends dorsally this process widens into a thin sheet culminating in a dorsal point. In lateral view, this process resembles a wing attached to the posterior end of the dorsal surface of the palate. The morphology of the quadrate ramus is very similar to that of Delorhynchus cifellii, including the presence of a tympanic flange, but cannot be compared to Colobomycter pholeter or Acleistorhinus pteroticus due to the current lack of information.
The right epipterygoid has also been preserved in association with this specimen, and is slightly disarticulated from the quadrate ramus of the pterygoid (Fig. 7B) This element consists of a ventral footplate which is broad, oval shaped and elongated anteroposteriorly, and a thin dorsal process with modest posterior curvature. The morphology of this element closely resembles the condition of Delorhynchus cifellii as described in Rowe, Bevitt & Reisz (2023).
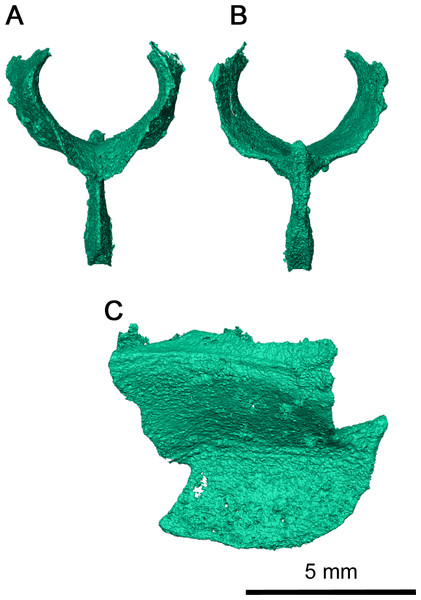
The sphenethmoid is a large, Y-shaped element in anterior and posterior view which has been very well-preserved (Fig. 8). In this specimen, it has shifted out of place, but is still relatively close to its articulating position on the ventral surface of the frontal (Figs. 3B and Fig. 4A). Dorsally, this element bifurcates into two processes curving medially which form the trough of the sphenethmoid (Fig. 6) (MacDougall et al., 2019). The morphology of the dorsal processes differs significantly from that seen Delorhynchus cifellii, where the dorsal processes do not curve medially (Rowe, Bevitt & Reisz, 2023). While the sphenethmoid of Colobomycter pholeter is not fully visible (Modesto & Reisz, 2008), it appears to possess a more modest curvature than Klastomycter conodentatus. In Feeserpeton, the dorsal processes of the sphenethmoid lack curvature and diverge laterally (MacDougall et al., 2019). The posterior end of the sphenethmoid curves downward into a short lip where the dorsal processes meet. Ventrally, the sphenethmoid has a long, straight, bladelike keel which widens slightly towards its posterior tip. This keel extends anteriorly past the dorsal processes and terminates in a sharp point above the contact between the dorsal processes.
Figure 8: Sphenethmoid of Klastomycter conodentatus.
(A) Anterior view, (B) posterior view and (C) left lateral view.Phylogenetic analysis
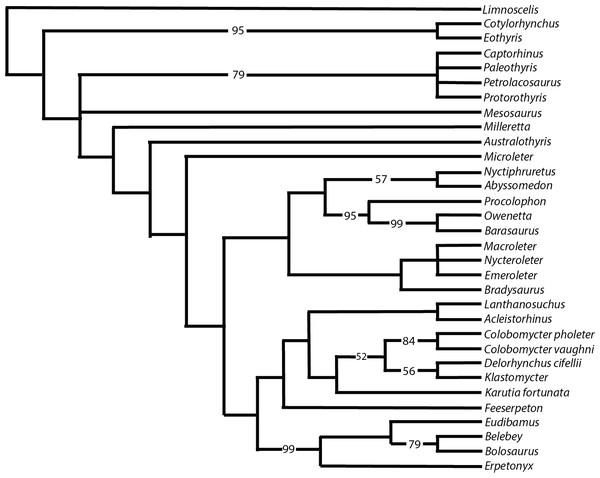
In order to determine the relationship of Klastomycter conodentatus to other members of Parareptilia, and acleistorhinids specifically, we added it to the data matrix of Rowe, Bevitt & Reisz (2023). The only change to that matrix was the addition of character states for Klastomycter conodentatus. Given the narrow scale of this study we did not include a broad range of taxa, or compare the results to Ford & Benson (2020) and Simöes et al. (2022). While it is important to evaluate the relationships between Parareptilia and other early amniotes, we do not consider this study to be a suitable place for such an undertaking.
Figure 9 shows the strict consensus of the 14 most parsimonious trees that were recovered following 73,882,182 rearrangements. Each tree has a length of 633 steps, a consistency index (CI) of 0.348, a retention index (RI) of 0.564, and a rescaled consistency index (RC) index of 0.196. Despite some of the more pronounced similarities of Klastomycter to Acleistorhinus, such as the lack of skull roof tuberosities, this new taxon is resolved as a sister taxon to Delorhynchus. Lanthanosuchus and Acleistorhinus form a clade as sister to the other so-called ‘acleistorhinids’, while Feeserpeton and the clade Bolosauridae are sister taxa to them. This pattern is the same as the previous analysis of parareptile interrelationships, but with Klastomycter closely related to Delorhynchus and Colobomycter. However, it must be noted that this pattern of relationship is weakly supported, and it only takes one extra step to collapse most basal parareptilian clades, with the exception of the clade formed by Klastomycter, Delorhynchus and Colobomycter. In view of that weakness, we refrain from changing the higher level designations until a better resolution to the patterns of relationships can be achieved. Thus, the family Acleistorhinidae was erected by Daly in 1969, when the parareptilian identity of Delorhynchus (Fox, 1962) and Colobomycter (Vaughn, 1958) were unknown. Similarly, the order and family designation of Lanthanosuchoidea (Efremov, 1946) was erected when the Acleistorhinidae were unknown.
Figure 9: Strict consensus of 14 most parsimonious cladograms of parareptile relationships.
Numbers represent majority-rule consensus values over 50%.Conclusions
‘Acleistorhinid’ diversity at the Richards Spur locality has continued to expand with the addition of this new taxon. Much of the diversity appears to be centered around the dentition, and its effect on the cranial anatomies of these small predators. As previously suggested, the Dolese Brothers Limestone Quarry locality and its cave deposits provide an unprecedented record of fine resource partitioning, with different but closely related taxa apparently using different types of dentition for food capture and possible processing. The latest member of this clade of small predators appears different from other acleistorhinids in the unusual conical shape of the teeth, but otherwise resemble most closely the more commonly found Delorhynchus.
In addition to the six ‘acleistorhinid’ taxa found at this locality, other small parareptiles also show startling dental diversity. Notable among these are three taxa of parareptiles, Bolosaurus, Microleter, and Abyssomedon. These taxa fall outside the ‘acleistorhinids’, and demonstrate that there was both an early diversification of this clade, and a broader diversity of early parareptiles not normally preserved (with the exception of Bolosauridae) in the Permo-Carboniferous sediments of Laurasia. This absence speaks to the overall limited knowledge of the fossil record of small amniotes in the initial stages of terrestrial vertebrate evolution, and that localities that represent natural traps provide a glimpse of this fundamental part of amniote history.
The Richards Spur locality underscores this lack of information, with the excellent preservation of unusual closely related taxa of small amniotes among the hundreds of thousands of known isolated and fragmentary bones, and the hundreds of skulls, partial skulls, and partial skeletons that have been recently uncovered. The new parareptile described here is a rare element of this community, as are some of the other parareptiles listed above, with Delorhynchus and Colobomycter being more frequently encountered in recently opened caves in Richards Spur.
This fine level of resource partitioning as recorded at the Richards Spur locality is not restricted to the parareptiles, but is also found among the captorhinid eureptiles, and is unknown elsewhere among Paleozoic terrestrial tetrapod localities. This is in great part because the cave deposits here, excavated as part of a large limestone quarry operation for nearly a century, represents by far the richest fossil locality for terrestrial tetrapods from the early Permian. The current evidence seems to indicate that other members of this paleocommunity preserved at this locality do not duplicate this level of taxic diversity, or this level of resource partitioning, although there are a couple of currently recognized small recumbirostran ‘microsaurs’. Similarly, the larger dissorophids are currently represented by three taxa, while trematopids appear to be restricted to a single taxon. New, ongoing research is likely to change this pattern, as new taxa are expected to be uncovered and incorporated into the expanding knowledge of this unique early Permian terrestrial tetrapod community.