The effect of aging on the ciliary muscle and its potential relationship with presbyopia: a literature review
- Published
- Accepted
- Received
- Academic Editor
- Nick Fogt
- Subject Areas
- Anatomy and Physiology, Ophthalmology
- Keywords
- Presbyopia, Ciliary muscle, Ciliary body, OCT, UBM, Aging, Lens, Vitreous body
- Copyright
- © 2024 Zuo et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. The effect of aging on the ciliary muscle and its potential relationship with presbyopia: a literature review. PeerJ 12:e18437 https://doi.org/10.7717/peerj.18437
Abstract
Background
The ciliary muscle is known to play a part in presbyopia, but the mechanism has not received a comprehensive review, which this study aims to achieve. We examined relevant articles published from 1975 through 2022 that explored various properties of the muscle and related tissues in humans and rhesus monkeys. These properties include geometry, elasticity, rigidity, and composition, and were studied using a range of imaging technologies, computer models, and surgical methods. We identified a notable age-related displacement of the ciliary muscle apex that is characterized by anterior and medial shifts, and hypothesized to be primarily attributed to the accrual of connective tissue and tension exerted by the thickening lens. Other factors could also contribute to the movement, particularly the “inward bowing” of the sclera. Another noteworthy observation is that while the ciliary muscle experiences increasing constraint with advancing age due to adjacent anatomical structures, its contractile capacity remains unaltered, alongside the sustained constancy in both the concentration of muscarinic receptors and their binding affinity. Overall, more studies on human ciliary muscle are needed, as it ages differently from that of monkeys’ ciliary muscle. These studies should explore other perspectives, including those regarding changes in the physical properties of the tissue and its relationship with other connected tissues.
Methodology
This literature review utilized a systematic methodology to identify and analyze pertinent studies of the presbyopia and ciliary muscles. The approach encompassed a thorough examination of available literature across different academic databases, such as PubMed, Embase, and Cochrane Library.
Results
Many studies have identified age-related thickening in the ciliary muscle and its potential causes, including the heightened deposition of connective tissues and traction exerted by the thickening lens. Notably, these inquiries culminated in the formulation of a geometric theory positing that the morphology of the ciliary muscle and its spatial relationship with adjacent structures exert pivotal influence over the tension exerted on zonular fibers.
Conclusion
The decline in the accommodative response of the muscle is prevalent in advanced age, with reduced mobility likely attributable to the increased stiffness of the Bruch’s Membrane-Choroid Complex (BMCC), where the tendons of the ciliary muscle insert, as well as the thickening and stiffening of the lens. Importantly, the ciliary muscle forces do not change with age.
Introduction
Presbyopia is a wide-spread condition that afflicts nearly every aging person on the planet. It is a refractive consequence of aging where patients lose their ability to focus their eyes on nearby objects. Such a loss of near vision means a great inconvenience and reduction in the quality of life. To tackle this challenge, different technologies that mainly focus on negating age-related changes in the lens have been developed (Wolffsohn & Davies, 2019). The most notable of these solutions is intraocular lens (IOL) technology, an area that has attracted considerable funding and attention from multidisciplinary experts. As a result, different types of IOLs, such as monofocal and multifocal IOLs, have been developed (Altun, 2020; Ang et al., 2020; Bianchi, 2020). Though they are able to improve the condition and even eliminate the use of glasses, they have their limitations and can cause new problems (Cho et al., 2022; Fernández et al., 2021; Sieburth & Chen, 2019). Some ardent proponents of IOL expect that the problem will be solved by future improvements of the technology, but there is a chance that better solutions lie elsewhere.
Given that the lens is only one of the components that may disrupt proper biomechanical mechanisms, it is imperative to direct greater attention towards developing treatment approaches that target other contributing factors. They have the potential to be incorporated into a customized regimen that best fits the patient if the causes of their presbyopia can be fully understood. However, in order to develop effective interventions, it is necessary to first examine how age affects the constituents of the eye.
One factor that demands attention is the ciliary muscle, which governs the tension of zonular fibers through contraction and thereby plays a vital role in accommodation, as suggested by Helmholtz’s theory of accommodation, a widely accepted and well supported theory (Bassnett, 2021; Hermans et al., 2007; Martin et al., 2005). Due to the unique structural location and large number of zonular fibers, current technology does not support direct measurement of the forces they generate, as it would disrupt the structure of the zonular fibers. Existing literature reports on the use of computer modeling to simulate the forces exerted by these fibers (Schachar, 2004). Goldberg (2011, 2015) have provided us with a computer model that reconstructs the accommodation process by rendering it in the form of computer animation. Knaus, Hipsley & Blemker (2021) took this a step further by devising a finite element model where parameters of the components can be adjusted to produce different results. Other theories have been proposed to explain the muscle’s role in presbyopia as it and its related structures are gradually changed by age. One theory that is gathering momentum is the geometric theory that places more weight on the shape-changing ability of the muscle than its contracting force. This theory will be discussed in subsequent sections.
Nevertheless, depicting the movement of the muscle can be difficult because the iris acts as a screen that completely renders the muscle invisible to ordinary observation methods. Though some studies evaded this problem by using iridectomized animals or albino patients (Baikoff et al., 2004; Croft et al., 2006), these are not widely applicable methods to visualize the ciliary muscle in human subjects. As a result, many imaging technologies have been employed to carry out the task. Two commonly employed techniques are ultrasound biomicroscopy (UBM) (Fernández-Vigo et al., 2022; Janssens et al., 2022) and anterior segment optical coherence tomography (ASOCT) (Ang et al., 2018); the former is usually preferred over the latter since it is unrestricted by the iris (Warjri & Senthil, 2022). In addition, magnetic resonance imaging (MRI), though not so popular, has also been used by researchers (Strenk et al., 1999; Strenk, Strenk & Guo, 2010). The research discussed in this review exclusively utilized one of these three methods. Through their application, the veil over the effects of aging on the ciliary muscle can be lifted.
In the following sections, before going into detail about age-related changes of the muscle, this paper reviews the basic structure of the ciliary body and muscle first. It approaches the problem from two widely explored perspectives (geometric and morphological changes) and changes in mobility and contractility. In the end, we provide a summary and deliberate on further directions.
Survey methodology
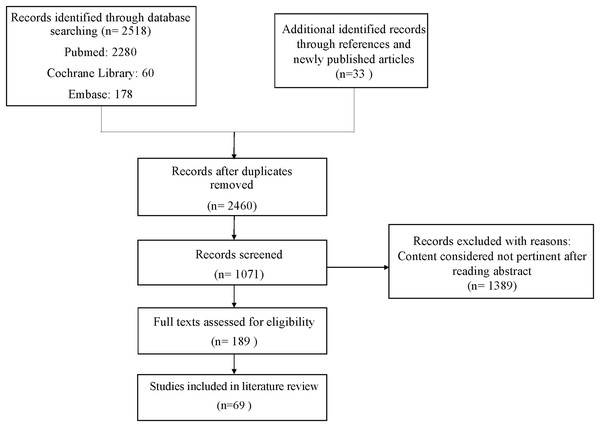
In January 2023, this study conducted searches across three databases: PubMed (MeSH), Embase, and the Cochrane Library. The search strategy utilized English-language search terms and combinations of them to explore articles related to “Ciliary muscle,” “Ciliary body,” “Zonules,” and “Ciliary Process,” combined with terms such as “Aging,” “Presbyopia,” “UBM,” “OCT,” and “aqueous humor.” Boolean operators (“+”, “AND”, and “OR”) were employed to refine the search results (Supplemental Material). The inclusion criteria for articles reviewed were as follows: (1) primary articles; (2) articles published in English or Chinese, irrespective of study design or publication date; and (3) studies considered most pertinent to this review. Identified articles underwent an initial screening to assess their relevance to the subject matter, and all pertinent articles were thoroughly reviewed. In this review, a total of 69 articles were included, and the screening process is illustrated in Fig. 1. To enhance the academic integrity and minimize plagiarism concerns, the search methodology was designed to reduce overlap with existing literature. Additionally, earlier literature reviews on the same topic were consulted to ensure comprehensive coverage of key themes without redundancy.
Figure 1: Flow chart.
The methodology used in this study has certain limitations and potential biases. The search was limited to English and Chinese articles from only three databases, which may have excluded relevant research from other languages or databases. Additionally, the inclusion of older articles may introduce outdated perspectives, and reliance on previous reviews could reinforce existing biases. Despite these limitations, every effort was made to cover key themes comprehensively.
The ciliary body and ciliary muscle
Ciliary body structure
The ciliary body is a circular structure positioned behind the iris, which is normally opaque and colored, thereby rendering direct observation of the structure without iridectomy highly challenging. The ciliary body stretches from the root of the iris, where the two meet and form a valley called ciliary sulcus, to the ora serrata, where the retina ends (Miesfeld & Brown, 2019; Warjri & Senthil, 2022). The length of the structure is usually measured from the scleral spur, a common reference point for carrying out measurements of the ciliary body, to the ora serrata (Sheppard & Davies, 2011). Moreover, it is worth mentioning that the ciliary body ring does not have a consistent length around the lens. On the temporal side, the ciliary body has a length of 5.6–6.3 mm, while on the nasal side the length is 4.6–5.2 mm.
With a cross-section resembling a right-angled triangle, the ciliary body can be divided into two parts: the anterior part called the pars plicata and the posterior part called the pars plana. In the pars plicata of typical individuals, there are some 70 ridges with abundant capillaries inside, and the ridges together form the ciliary processes (CP). Between the ridges are furrows where zonular fibers, which suspend the lens, attach to the processes. The CP are lined by two layers of epithelial cells, with the non-pigmented layer on the outside and the pigmented one on the inside. Together, they are in charge of the production of aqueous humor, an important fluid that supplies nutrients to and removes wastes from avascular tissues and keeps intraocular components in position by maintaining intraocular pressure (IOP). In contrast, though the pars plana is covered by zonular fibers inserted into its posterior segment, no significant functions have yet been discovered related to the pars plana, which is less vascularized and, as indicated by its name, flat. As a result, the region is regarded as a safety area through which surgical procedures like pars plana vitrectomy access the inside of the eyeball.
Ciliary muscle structure and function
With its inner side separated from the double layers of epithelia by laminae of collagen fibers, and capillaries, the ciliary muscle comprises most of the ciliary stroma. In addition, its outer side is connected to the inner sclera via a lamina called supraciliaris. The anterior tendons of the muscle attach it to the scleral spur and trabecular meshwork, while in its posterior segment, tendons connect it to the Bruch’s membrane, a structure that gives elasticity to the choroid.
The ciliary muscle is a smooth muscle that consists of three types of muscle fibers that differ from each other by the directions they run in Bassnett (2021). The longitudinal, or meridional, fibers run from the anterior to the posterior part of the eye. They form the outermost layer of the muscle, which is in juxtaposition to the inner surface of the sclera. In the front, they attach to the sclera spur and trabecular meshwork, while in the rear they merge with the stroma of the choroid. The radial fibers run obliquely, from medial to lateral, and from the CP to the chamber angle. As a result, they form a reticular pattern that is present throughout the middle of the ciliary muscle. The circular fibers run perpendicular to the longitudinal ones, and as the fibers go around the globe, they form a circular shape. As the innermost part of the ciliary muscle, circular fibers are the closest to the apex of the CP (Knaus, Hipsley & Blemker, 2024).
There are two neural pathways associated with accommodation that innervate the ciliary muscle: the sympathetic innervation, which is responsible for disaccommodation of the muscle, and the parasympathetic innervation, which is the major innervation responsible for triggering accommodative contraction of the muscle. Starting from the diencephalon, the sympathetic fibers proceed down the spinal cord until they pass through the C8-T2 segments into the cervical sympathetic chain and then synapse in the superior cervical ganglion. Following that, axons of postganglionic neurons leave the region to form the sympathetic carotid plexus, pass through the ciliary ganglion, and pierce the eye via short ciliary nerves. There are also other sympathetic fibers navigating the long ciliary nerves and optic canal. The parasympathetic pathway, however, begins as preganglionic fibers issuing from the Edinger–Westphal nucleus, traveling inside the oculomotor nerve, arriving at the ciliary ganglion, and synapsing in it. The pathway then continues in the form of axons of postganglionic neurons that leave the ganglion, enter the eyeball, and supply the ciliary muscle through the short ciliary nerves (McDougal & Gamlin, 2015).
The ciliary muscle plays a crucial part in accommodation. According to Helmholtz’s theory, when the ciliary muscle is in a relaxed state, zonular fibers that suspend the lens are tensioned and exert a stretching force on the lens that makes it less rounded. The eye is in a disaccommodated state at this stage, allowing it to clearly focus on distant objects, reflecting the dynamic shift in lens shape from a state optimized for near vision back to a state optimized for far vision (Glasser, 2008). On the other hand, when the ciliary muscle contracts, the tension on the zonular fibers is released, so the force that pulls on the lens is no more. As a result, the lens is more rounded. This process, called accommodation, is where the eye tries to focus on nearer objects by changing the power of the lens, which is decided by its roundness. It is widely held that during the accommodation of a normal eye, the ciliary muscle exhibits a forward and inward shift in mass, a shift that can mostly be attributed to the movement of the longitudinal and circular fibers (Croft et al., 2013b; Goldberg, 2015).
Apart from accommodation, the ciliary muscle can also have an impact on IOP because it affects the outflow of aqueous humor. There are two routes that the fluid takes to leave the eye. One is the conventional pathway where the fluid enters from the trabecular meshwork at the limbus into the Schlemm’s canal, where it is drained into veins. Most aqueous humor drains into this pathway. The other way that aqueous humor leaves the eye is by filtering through the ciliary muscle, which is called the unconventional pathway. Since connective tissues between muscle bundles are loose, the fluid can seep through the tissues into the supraciliary and suprachoroidal spaces before passing beyond the sclera (Bill, 1977; Goel et al., 2010).
The ciliary muscle is able to influence both aqueous humor outflow pathways. As the muscle is connected to the trabecular meshwork anteriorly, it changes the geometry of the meshwork when it contracts, making it less tightly packed, and so its resistance to outflow is reduced. Conversely, when the muscle relaxes, the elasticity of the meshwork returns it to its original form, resulting in increased restriction to the outflow (Bill, 1977; Goel et al., 2010). In the second pathway, which is also called the uveoscleral pathway, the contraction of the ciliary muscle reduces the permeability of the tissue and diminishes the outflow, with the majority of the effect being exerted on the main pathway (Bill & Phillips, 1971). Given this, it is possible that age-related changes in the ciliary muscle are not only responsible for presbyopia, but also for other diseases—glaucoma, for example. If such a link is real, studies about other diseases associated with the ciliary muscle could in some ways increase our understanding about the muscle and its relationship with presbyopia (Chen et al., 2021; Kaufman, Lütjen Drecoll & Croft, 2019; Naroo et al., 2024; Pallikaris et al., 2005).
Age-related geometric and morphological changes of the ciliary muscle
The eye is a sophisticated and delicate optic system where a tiny alteration in the shape or relative position of a structure can make a huge difference in the image we perceive. When the eye ages, changes happen in its constituents, and consequently, such alterations occur can occur to components inside and so affect its function. The lens, for example, thickens as we age, and so its shape and relative position with respect to the zonules and ciliary muscle become different (Pardue & Sivak, 2000). Theories have been proposed to explain the relationship between these changes and presbyopia (Koretz & Handelman, 1988; Koretz, Handelman & Brown, 1984; Strenk, Strenk & Koretz, 2005; Tamm, Tamm & Rohen, 1992). Tamm et al. (1991) even postulated that the major factor dictating the force on the lens is the geometry and arrangement of the ciliary muscle (Tamm, Tamm & Rohen, 1992). The same attention should be paid to examining age-related geometric and morphological changes of the ciliary muscle, since the implications could be huge.
Currently, researchers focus almost entirely on the anterior segment of the ciliary muscle. Many studies have identified that with age, the ciliary muscle in human changes to a more centripetal position. There is also a diminishing ciliary muscle ring diameter (Pardue & Sivak, 2000; Richdale et al., 2016; Strenk et al., 1999; Strenk, Strenk & Guo, 2010; Tamm, Tamm & Rohen, 1992). Such movement makes the disaccommodated ciliary muscle in older eyes somewhat resemble its accommodated counterpart in younger eyes. Using magnetic resonance imaging (MRI), Strenk et al. (1999) measured the ciliary muscle ring diameter directly in images where the muscle could be identified as a hypointense pixel cluster with a triangular shape. They found a clear trend that ring diameter shortened as age increased. Moreover, to determine the relation between age and the position of the ciliary muscle, Richdale et al. (2016) and Strenk, Strenk & Guo (2010) used Anterior Segment Optical Coherence Tomography (ASOCT) and MRI, respectively, to look at the anteroposterior position of the ciliary muscle apex and width of the muscle. In both studies, older subjects exhibited a forward movement of the ciliary muscle apex and an increase in its thickness. Although potential challenges such as artifacts and distortion caused by subject motion, as well as the strong correlation between the antero-inward movement of the ciliary muscle and aging, were confirmed.
Pardue & Sivak (2000) and Tamm, Tamm & Rohen (1992) drew their conclusions from postmortem examinations of donated eyes. Admittedly, it is possible that the two studies observed structures that might have undergone changes after donors’ deaths or during operations. The ischemic condition, for example, might have rendered the ciliary muscle less responsive to pharmacologic agents. Additionally, the loss of structural integrity might also have affected the shape of tissues. Nevertheless, the studies did provide more insight into the cause of the movement by offering a histological view of the ciliary muscle. Both found a significant increase in the connective tissue inside the ciliary muscle area in older individuals. This increase occurs most notably in the circular and reticular portions while being insignificant in the longitudinal portion. As more connective tissue grows between muscle bundles, the bundles grow further apart, which increases the muscle area. This thickening process of the muscle could at least partially explain why the muscle ring becomes smaller with age (Fernández-Vigo et al., 2022).
In addition to studies that directly measured the ciliary muscle, other studies used an indirect method by focusing on the circumlental space (CLS), which is the space between CP and the equatorial edge of the lens (Croft et al., 2006, 2013a; Glasser et al., 2001). These studies observed decreasing CLS in older subjects. Since the lens equator itself does not grow in diameter with age (Sakabe et al., 1998; Strenk et al., 1999), the only way for the space to gradually narrow is by CP moving closer to the lens. Such displacement could partially be attributed to age-related changes in the sclera, which undergoes crosslinking and stiffening over time (Smewing, 2020). This increased rigidity restricts biomechanical movements and prevents fibril sliding and inward movement (Croft et al., 2013b; Detorakis et al., 2010; Detorakis & Pallikaris, 2013). The “inward bowing” of the sclera at the limbus has been documented by Croft et al. (2013b). Moreover, diminishing CLS could indicate that the ciliary muscle or CP thicken with age, or both. According to the conventional electron microscope study by Marshall et al. (1992), stromal fibrosis happens both to the stroma in CP and those between ciliary muscle bundles. The parts in the muscle that are most affected are the oblique and circular portions, in which patchy thickening are found. Nonetheless, this thickening of stroma between ciliary muscle bundles is where humans and rhesus monkeys differ, because in aged eyes of rhesus monkeys, intramuscular connective tissue was only observed to have increased a tiny amount. This suggests that although the eyes of rhesus monkeys are morphologically and physiologically similar to those of humans and both species develop presbyopia at the same stage of life, the mechanism behind presbyopia in rhesus monkeys could well be different.
However, even though connective tissue could be partially responsible for a diminishing ciliary muscle ring diameter, the major cause underlying the phenomenon is very likely to be an aging lens. Strenk et al. (1999) found that, to the contrary of phakic subjects who showed a centripetal age-related displacement of the ciliary muscle, pseudphakic subjects had their ciliary muscles recovered to a position similar to that of the muscle in a 29-year-old phakic eye. Wasilewski et al. (2008) carried out intracapsular lens extraction (ICLE), a surgery that removes the lens and its capsule and therefore cuts the zonule, and extracapsular lens extraction (ECLE), a procedure that removes only lens content, on rhesus monkeys. Compared to the control, the monkeys receiving ECLE exhibited only a small decrease in their resting ciliary body thickness while those receiving ICLE had much thinner ciliary muscle profiles, especially in the anterior portion, and posteriorly positioned muscle apices. These two studies attest that the lens is pulling on the ciliary muscle via the zonule, and a thickened lens causes the muscle to be displaced centripetally. Moreover, some research has found that the attachment of the anterior zonules moves away from the lens equator as the lens ages (Farnsworth & Shyne, 1979; Sakabe et al., 1998). Consequently, such movement may consequently exert an excessive pulling force on the ciliary muscle, forcing it to move. Overall, these studies provided evidence that backs a geometric theory: as the aging lens grows in thickness, the insertion ring of zonules moves forward away from the lens equator and creates a drag on the ciliary body, which in turn causes an antero-inward movement of the ciliary muscle.
The implications of such a movement could be huge. Changes in the shape and position of the muscle and consequentially altered geometric relationships between the muscle and related components may greatly affect the outcome of accommodation and disaccommodation. However, more proof is needed. Computer models could offer some help since they are good at reconstructing the whole process of accommodation, which current in vivo and in vitro methods are insufficient at achieving. Furthermore, since the ciliary muscle and processes bulk up as the eye ages, even if the muscle increased by the same amount in thickness during accommodation as its younger version would, it would not produce the same level of change to its geometry (Knaus, Hipsley & Blemker, 2021). Additionally, there exists an age-related loss in apex thickening as reported by Croft et al. (2013a). This is further supported by the findings of Detorakis & Pallikaris (2013), who demonstrated that ocular rigidity correlates with reduced accommodation with age. As a result, when this factor combines with the antero-inward displacement of the muscle, the zonules may not relax enough due to insufficient change in the configuration of the mechanical system composed of the lens, zonules, and the ciliary muscle. This can produce a pull on the lens that prevents it from rounding up, so presbyopia may arise.
Age-related changes in mobility and contractility
Definition of mobility and contractility
As mentioned above, the ciliary muscle, a key component in accommodation, controls the process via contraction, so whether it maintains the same ability to contract at an old age is worthy of particular attention. Some studies on humans and rhesus monkeys have identified a smaller amount of muscle contraction in old people. Croft et al. (2013a) compared the thickening of ciliary muscle apex during accommodation in old and young people, and they revealed that the accommodative apex thickening reduced significantly with age (Croft et al., 2013a). Another study carried out by Xie et al. (2022) increased accommodative stimuli step by step and recorded alterations in Maximum Ciliary Muscle Thickness (CMTMAX) and the span between the scleral spur and the position with maximum ciliary muscle thickness (SSMAX). CMTMAX thickening, according to the results, leveled off in the middle-aged group at three diopters. Meanwhile, the shortening of SSMAX also plateaued at 3D in the same group. In contrast, in the younger group, the two parameters kept increasing as the stimuli stepped up. In another study, Tamm et al. (1992) administered atropine and pilocarpine to rhesus monkeys and observed changes in biometrics of ciliary muscles administered with the two agents. Similarly, they found weakened ciliary muscle contraction in older eyes.
However, before diving into a search for root causes of this age-related change wrought by senility, it is crucial to distinguish between two different factors that may determine the extent to which the muscle can contract: mobility and contractility. The former measures how much latitude to contract is granted to the muscle by the surrounding structures connecting to it, and the latter is an indicator of the ability of the muscle to contract. If mobility is low, the muscle is restricted by other components and thus cannot contract as it normally would. Also, if the muscle loses its contractility, it will not produce the same amount of force to sustain the same level of contraction.
Age-related changes in mobility
Since the mobility of the muscle is dependent on components outside, a clear understanding of how the muscle connects to its surrounding structures is a prerequisite for further studies of age-related changes in mobility.
In the front, the muscle is anchored to the scleral spur through elastic tendons and is indirectly connected to the lens via zonular fibers attaching to the internal limiting membrane that covers the nonpigmented epithelial cells in the valleys between the CP (McCulloch, 1954). Studies concerning the anterior parts where the muscle connects have been focused on as a cause for the loss of ciliary muscle mobility in an aging lens. Having rhesus monkeys as their subjects, Croft et al. (2008) collected data about CP movement under electric stimulation in younger and older groups before and after extracapsular lens extraction (ECLE) (Croft et al., 2008). According to the results, young eyes did not show much of a change in the accommodative CP movement after the surgery, while the CP movement in older eyes, which initially had a much lower level of CP movement, increased to close to that of the pre-operative young eyes. These results suggest that increased lens volume contributes to the restriction of ciliary muscle in an aging eye. In addition, other studies about cataract extraction may also shed a light on the problem. Park, Yun & Kee (2008) and Fayed (2017) selected patients over 35 years old who suffered from cataracts as their subjects and used UBM to visualize accommodative ciliary body movement in them before and after the surgery of cataract extraction and IOL implantation. Both studies confirmed that phacoemulsification surgery helped recover a significant level of centripetal movement of the ciliary muscle. In light of the aforementioned geometric theory, a possible decrease in lens thickness due to the surgery proposed by Fayed (2017) may well be the reason of the recovery. Therefore, it can be proposed that as the lens ages, its increasing thickness is likely to reduce the excursion of the ciliary muscle.
On the other hand, the posterior fibers of the ciliary muscle are attached to Bruch’s membrane of the choroid through elastic tendons at the ora serrata, where the pars plana zonules (PPZ) form the valleys between the ciliary processes and connect to the ciliary epithelium (McCulloch, 1954). In addition to this group of zonules, the intermediate vitreous zonules (iVZ) also originate from these valleys, run posteriorly in the cleft between the PPZ and anterior hyaloid, split at the ora serrata into fine fibrils that merge with the anterior hyaloid, and connect it to the PPZ (Croft et al., 2016; Lütjen-Drecoll et al., 2010). Another newly discovered group of zonules termed the posterior vitreous zonule insertion zone to the lens equator (PVZ INS-LE), however, attaches to the posterior lens equator directly, though it courses in the same direction as the former two groups: towards the posterior insertion zone at the ora serrata (Croft et al., 2016). Apart from the choroid and zonules, one other structure that connects to the ciliary body is the vitreous body, whose anterior hyaloid fuses with the surface of the ciliary body at the ora serrata (McCulloch, 1954).
A stiffening choroid could be a potential cause for decreasing mobility of the muscle. According to observations by Croft, Lütjen-Drecoll & Kaufman (2017), when the muscle contracts during accommodation, it pulls the choroid forward. Due to its elasticity, the choroid is pliable, and as force is applied to the back of the eye, it produces a centrifugal movement of the choroid with the optic nerve head as the center of the movement (Croft, Lütjen-Drecoll & Kaufman, 2017; Croft et al., 2022b; Marshall et al., 1992). As the muscle is connected to the elastic choroid, changes in the structure’s pliability under contractile force should make it easier for the muscle to contract. On the other hand, if the structure loses its elasticity, a drag force may resist the ciliary muscle forces. Ugarte, Hussain & Marshall (2006) and Graebel & van Alphen (1977) used their own devices to apply forces to strips of the choroid and obtained the stress–strain relation of the material. Their results suggest that the elasticity of the choroid decreases significantly with age. Accordingly, the decrease could eventually make it harder for the muscle to contract. However, restriction from the choroid possibly plays a major role in lowering ciliary muscle mobility. According to Tamm et al. (1992), when the eyes of young and old rhesus monkeys were bisected meridionally after being administered with pilocarpine, the contractile response of the ciliary muscle to the agent was lost in old eyes. However, this loss was only exhibited in the middle parts where the choroid was intact (Tamm et al., 1992). At the site where the choroid was detached from the sclera, the effect of pilocarpine was retained, and no discernible difference in inner apical position was observed among the three age groups. Though eyes of rhesus monkeys may differ from those of humans in some respects, this study indicates that a stiffer choroid may be a major cause of decreasing mobility of the muscle. Moreover, choroid and the elastic tendons that fasten the choroid to ciliary muscle have been reported to become more rigid by growing thicker with age, potentially further diminishing the mobility of the muscle (Tamm et al., 1991).
Another potential factor that may increase restriction on the ciliary muscle is an aging vitreous body. Though anatomically it may look like a simple knob of gel, this component is essentially made of cisterns, which are individual chambers that are filled with gel and framed by collagen fibers (Jongebloed & Worst, 1987). When the component ages, the collagen fibers start to stick together and become stiff, and vitreous liquefaction, which further increases stiffness by increasing the liquid amount inside, occurs. Consequently, a stiffened vitreous body probably does not respond to pulling force as well as before and can even generate more resistance against the force. As reported by Croft et al. (2022a, 2013b), the peripheral vitreous in rhesus monkeys is pulled forward during accommodation by the vitreous zonules at regions where the two structures fuse together, while the anterior hyaloid bows backward behind the lens. However, the movements decrease noticeably with age (Croft et al., 2022a, 2013b). Overall, these results suggest that a stiffened vitreous body also contributes to age-related reduction in the mobility of the ciliary muscle.
Apart from exterior causes, the decrease in mobility could come from changes within the muscle as well. Hyalinization, a process that turns stroma into homogeneous translucent materials, has been reported to be a prevalent condition in humans over 50 years old, and takes place in areas from the CP stroma to the stroma separating the reticular portion and the circular portion (Croft et al., 2013b; Tamm, Tamm & Rohen, 1992). It is possible that this process contributes to the development of presbyopia by altering physical properties of the stroma, making it more rigid. Former studies have indicated that the circular fibers, which are interspersed with connective tissue, produce most of the antero-inward movement of the muscle (Lewis et al., 2012; Lossing et al., 2012; Mohamed Farouk et al., 2018). An increase in the rigidity of connective tissue could suppress that movement and help bring about presbyopia.
On the whole, the ciliary muscle becomes more and more restricted when it, and other structures of the eye undergo age-related changes. An ever-thickening lens in the anterior chamber and an ever-hardening vitreous body in the posterior chamber, or the muscle itself, may be responsible for that. The consequential restriction, when strong enough, will deprive the muscle of the ability to produce enough geometric change that is needed to loosen up zonular fibers and release tension on the lens, so presbyopia occurs.
Age-related morphological changes
As individuals age, the morphology of the ciliary muscle undergoes notable changes, particularly in fiber type composition (Flügel, Lütjen-Drecoll & Bárány, 1990). The proportion of Type I fibers (slow-twitch muscle fibers typically associated with endurance activities) increases with age, while the proportion of Type II fibers (fast-twitch muscle fibers associated with rapid, short-duration activities) decreases. This shift in fiber type may adapt the ciliary muscle to meet the demands for sustained rather than instantaneous contraction. Research by Sheppard & Davies (2010), employing AS-OCT technology, provides new insights into this adaptation. Their observations revealed that with increased accommodative demand, the anterior portion of the ciliary muscle exhibits significant contraction-induced shortening and thickening of the anterior muscle body (Sheppard & Davies, 2010). This suggests that, with aging, the ciliary muscle undergoes structural adaptations to accommodate new functional requirements. Additionally, changes in fiber type have led to more pronounced fiber grouping within the ciliary muscle, further reflecting its adaptive structural changes. Sheppard & Davies (2011) further reported that the length of the anterior ciliary muscle in emmetropic eyes significantly decreases with age, whereas its maximum width increases (Sheppard & Davies, 2011). These alterations are likely closely associated with changes in muscle fiber types.
Furthermore, the overall mass of the ciliary muscle increases, potentially due to an increase in the number of muscle fibers and muscle volume. This phenomenon is considered a compensatory mechanism aimed at preserving contraction capability in the aging ciliary muscle. However, further research is required to determine whether these morphological changes can fully offset the functional decline associated with aging.
Age-related physiological changes
The ciliary muscle may undergo physiological changes with aging, including alterations in muscle fiber composition, hypertrophy, and atrophy (Tamm, Tamm & Rohen, 1992). While earlier studies, such as those by Tamm, Tamm & Rohen (1992), suggested that both the longitudinal and reticular portions of the ciliary muscle might experience atrophy with age, more recent research indicates that the ciliary muscle may hypertrophy to generate greater force required for accommodating the aging lens. Additionally, as the sclera becomes stiffer and the choroid becomes more relaxed, these changes may prompt the ciliary muscle to increase its mass to adapt to the structural changes in the aging eye.
Tamm, Tamm & Rohen (1992) observed that the area of the longitudinal portion decreased by more than half as the age of the subjects increased from 30 to 80 years. More pronounced atrophy may occur in the reticular portion, where the proportion of connective tissue increased from 20% in subjects aged 30 to 40 years to 50% in those aged 50 to 60 years, with a concurrent decrease in the area of this portion. However, Sheppard & Davies (2010) used anterior segment optical coherence tomography (AS-OCT) to observe a significant increase in the thickness of the anterior part of the ciliary muscle (CM25) during accommodation, suggesting that the ciliary muscle might hypertrophy to meet the demands of accommodation. Furthermore, Hipsley & Colbert (2022) proposed that although the ciliary muscle maintains or even increases its contractile force with age, the development of presbyopia may be related to biomechanical changes in the ciliary muscle and other ocular structures.
While Tamm, Tamm & Rohen (1992) reported an increase in the area of the circular portion with age, Marshall et al. (1992) using immunoelectron microscopy found significant atrophy in both the reticular and circular portions (Croft et al., 2013b). The atrophy of the circular portion, which is responsible for most of the ciliary muscle’s accommodative movement, may reduce contractile force and contribute to presbyopia. These findings suggest that hypertrophy and atrophy of the ciliary muscle may represent different aspects of presbyopia development, highlighting the need for further research to understand how these changes collectively impact accommodative ability and the progression of presbyopia.
Age-related contractile changes
The contraction force of the ciliary muscle, or the muscle’s ability to contract, is influenced by various factors, including the proportion of muscle cells, the number of muscarinic binding sites, and the activities of choline acetyltransferase (ChAT) and acetylcholinesterase (AChE). Before exploring how aging affects these specific factors, it is crucial to understand how aging impacts the overall function of the ciliary muscle.
Poyer, Kaufman & Flugel (1993) studied the contraction force of aged ciliary muscle through in vitro experiments, measuring the contraction responses of monkey ciliary muscle strips after administering carbachol and aminoethylacetic acid. Although in vivo experiments indicate that with aging, the contraction response of live monkey ciliary muscle to muscarinic agents weakens, in vitro tests did not reveal a significant relationship between contraction force and age. This finding aligns with results from cataract surgery, which typically restores much or all of the ciliary muscle’s contraction force, suggesting that even in old age, the ciliary muscle retains significant contraction potential (Fayed, 2017; Park, Yun & Kee, 2008). However, research by Pardue & Sivak (2000) indicates that although the ciliary muscle responds to drug treatments at all ages, a decrease in ciliary muscle length may be associated with a potential decline in contraction force (Pardue & Sivak, 2000). This echoes Poyer, Kaufman & Flugel’s (1993) findings, suggesting that while the aged ciliary muscle still retains some contraction force, it may be affected by aging. With increasing age, the response of the ciliary muscle to electrical stimulation and muscarinic cholinergic agonists (such as pilocarpine) may weaken, possibly related to degenerative changes in intramuscular nerves, changes in muscarinic receptor concentrations, or reduced receptor binding affinity.
Although Poyer, Kaufman & Flugel (1993) did not find significant age-related changes in contraction force, research by Lütjen-Drecoll et al. (2010) observed significant degenerative changes in muscle cells and myelinated nerve fibers in monkey ciliary muscles using optical and electron microscopy. These changes were closely related to a decline in accommodative amplitude and stabilized after the age of 25. In contrast, no significant changes in muscarinic receptor concentration or receptor binding affinity were observed (Gabelt, Kaufman & Polansky, 1990). However, since the aging process of the human ciliary muscle may differ from that of monkeys, these results might not be directly applicable to humans. In exploring the impact of aging on ocular accommodative ability, Hipsley & Colbert (2022) proposed a new model that provides a more comprehensive perspective. They noted that with increasing age, changes occur in the biomechanical and physiological functions of the eye, not limited to the lens but also involving the ciliary muscle, vitreous body, sclera, and other ocular structures (Hipsley & Colbert, 2022). Specifically, they emphasized that although the ciliary muscle can maintain its contraction force even in old age, the decline in accommodative ability may be related to biomechanical dysfunctions of the entire ocular accommodative apparatus. Furthermore, Hipsley and Colbert’s study indicated that with age, ocular structures might undergo cumulative damage, such as lens hardening due to oxidative stress and advanced glycation end-products (AGEs), increased scleral biomechanical stiffness, and decreased elasticity of the ciliary muscle tendons and Bruch’s membrane-choroid complex (BMCC). These changes collectively contribute to a reduction in accommodative amplitude and ultimately affect the eye’s accommodative function (DRoF). Although current research has not reached a definitive conclusion regarding age-related changes in contraction force, existing evidence suggests that the ciliary muscle retains a certain degree of function during aging. Future research on the human eye is needed to further validate this phenomenon.
The contraction force of the ciliary muscle may not significantly decrease in the elderly or may only decrease slightly; therefore, its role in the decline of accommodative function in presbyopic patients may be relatively minor.
Summary and future directions
Although more studies are needed to validate some discoveries and theories concerning the aging process of the ciliary muscle and to reveal more unknown sides of this process, several questions can be asked. First, there are significant age-related changes in the geometry and morphology of the ciliary muscle, which cause it to become displaced forward and inward. Such displacement can be represented by the thickening and forward movement of the ciliary muscle apex and are explained by an increase in connective tissue and the pulling force from the thickened lens. It is worth noting that the age-related narrowing of the circumlental space (CLS) should not be interpreted solely as the consequence of the antero-inward movement of the muscle. Other factors could also contribute to this movement, especially the “inward bowing” of the sclera. Further studies are needed to confirm the existence of age-related narrowing of the CLS and investigate its potential relationship with accommodation and presbyopia.
However, whether the geometric and morphological changes have substantial influence on accommodation remains unclear. The question may be better answered by computer models, as they excel in controlling variables and visualizing the process. This could also prove to be a direction for future research.
Another issue brought about by age is the irresponsiveness of the ciliary muscle to stimuli. The major reason for this is the age-related loss in the mobility of the muscle, caused by a stiffening choroid, hardening vitreous body, thickening lens, and stiffening of the sclera. Hyalinization in the muscle tissue is possibly another culprit, but there are currently no studies on how it affects the physical properties of the ciliary muscle tissue, making it an area worth further investigation. Additionally, as the ciliary muscle mass advances with age, the length of the pars plana increases (Pardue & Sivak, 2000; Richdale et al., 2016). This may intriguingly affect the tightness of posterior components and consequently influence the mobility of the ciliary muscle.
In contrast to the decline in mobility, no considerable decline in contractility has been found, though the atrophy of myocytes has been observed. In addition, evidence from rhesus monkeys suggests that the concentration of muscarinic receptors and binding affinity of the receptors remain unchanged at an old age. Nevertheless, it should be noted that experiments about contractility have nearly all been carried out on rhesus monkeys, whose eyes are similar to those of humans but age in a different way, as mentioned formerly (Lütjen-Drecoll, Tamm & Kaufman, 1988). Therefore, more experiments conducted on the human ciliary muscle are needed.
One other overlooked potential direction is not directly related to presbyopia. As previously mentioned, age-related changes in the muscle can be the common underlying mechanism for presbyopia and other diseases like glaucoma. This has been discussed and supported by Kaufman, Lütjen Drecoll & Croft (2019). Exploring the insights gained from studying other diseases could potentially increase our understanding of presbyopia and its relationship to the ciliary muscle.
Conclusion
There are complex interactions among all of the anatomical components of the eye, and the ciliary muscle, a vital component of accommodation, is not immune to aging. Therefore, the aging of the ciliary muscle may well be a trigger to presbyopia. Studies about the aging process of the muscle mainly center on changes in its geometry, its relative position to related components, mobility, and contractility. Though it hides behind the opaque iris, imaging technologies such as UBM have been employed to visualize it and its accommodative movement. Moreover, when a detailed examination of the muscle’s fine structures is needed, light and electron microscopy are valuable tools for researchers. These technologies allow for the investigation of the muscle’s fine structures, including the increase in connective tissues and the effects of lens thickening. More importantly, the studies discussed have contributed to various theories about the ciliary muscle’s role, including geometric theory, lenticular theory, capsular theory, and zonular theory. These theories collectively explore how the shape of the ciliary muscle and its interactions with other components influence the tension of zonular fibers. In addition, other studies have confirmed that most of the accommodative response of the muscle are lost at an advanced age and the loss of mobility is the main culprit, which could be caused by a thickening lens and stiffening choroid. In contrast, the muscle’s contractility remains relatively stable throughout the lifespan. Future studies, particularly those focusing on human ciliary muscles, will shed more light on the situation by determining whether previous findings in rhesus monkeys can be extrapolated to humans and uncovering more unknown factors contributing to presbyopia. Moreover, incorporating computer-based studies or in-silico models could be beneficial by providing further insights into the aging process of the ciliary muscle and its implications for presbyopia (Cabeza-Gil et al., 2022; Goldberg, 2015; Knaus, Hipsley & Blemker, 2021, 2024).