Hydrogen gas inhalation prior to high-intensity training reduces attenuation of nitric oxide bioavailability in male rugby players
- Published
- Accepted
- Received
- Academic Editor
- Philip Reno
- Subject Areas
- Anatomy and Physiology, Kinesiology, Sports Injury, Sports Medicine
- Keywords
- Adaptation, Hydrogen, Nitric oxide, Oxidative stress, Sports training
- Copyright
- © 2024 Zhao et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Hydrogen gas inhalation prior to high-intensity training reduces attenuation of nitric oxide bioavailability in male rugby players. PeerJ 12:e18503 https://doi.org/10.7717/peerj.18503
Abstract
Background
Inhalation of hydrogen gas (H2) as an antioxidant supplement may alleviate exercise-induced oxidative damage and protect post-exercise hydrogen peroxide signaling, which may help mediate beneficial exercise adaptation. The aims of this study were to determine the effects of H2 inhalation on plasma nitric oxide (NO) level and its synthesis precursor in professional athletes.
Methods
A randomized, placebo-controlled, double-blind, crossover trial was conducted with professional male rugby players for 3 weeks. Participants underwent 1 week of H2 supplementation and 1 week of placebo treatment prior to daily sessions of high-intensity exercise training, separated by 1 week of low-intensity training as a washout.
Results
Two-way (supplementation and time) repeated-measures analyses of variance showed that NO, L-arginine, and tetrahydrobiopterin levels in the H2 inhalation group were significantly higher than those in the placebo group after exercise (D6) and remained higher after 24 h of rest (D7). Levels of hydroxydeoxyguanosine and interleukin 6 were lower in the H2 inhalation week than in the placebo week on D6 and D7. In addition, total antioxidant levels were significantly higher with H2 inhalation than with placebo.
Significance
These results suggest that H2 inhalation helps to maintain NO signaling after exercise and to alleviate inflammation and oxidative stress induced by high-intensity exercise training in professional athletes.
Introduction
A key strategy for improving physical performance in sports and exercise training is based on the overload principle (Impellizzeri et al., 2020; Zaryski & Smith, 2005). However, overloading is often accompanied by inflammation and oxidative stress that may cause tissue damage and fatigue (Markus et al., 2021; Owens et al., 2019; Powers & Jackson, 2008). One approach to help athletes maintain a high level of performance and reduce fatigue and injury is supplementation with antioxidants. It has been reported in the literature that vitamin C and E supplementation attenuates the increase in mitochondrial protein induced by endurance training, thus attenuating the adaptation to exercise (Paulsen et al., 2014). Supplementation with the antioxidant allopurinol has been reported to reduce oxidative stress but impair beneficial cellular defenses and adaptations to exercise in rats (Gomez-Cabrera et al., 2005). However, exogenous antioxidants blunt cell signaling responses that are favorable to exercise adaptations (Chang et al., 2007; Shannon et al., 2022; Suzuki et al., 2020), and may, therefore, impede training effects.
Previous studies have shown that exercise-induced increases in Superoxide anion (O2−), nitric oxide (NO), and hydrogen peroxide (H2O2) production can stimulate cell-cell adhesion, cell signaling, vasoregulation and fibroblast proliferation, and the expression of antioxidant enzymes (Cadenas, 2018; Martinez de Toda et al., 2019; Merry & Ristow, 2016; Powers et al., 2020; Powers & Jackson, 2008; Sies & Jones, 2020). Although vitamin C supplementation inhibits superoxide production and reduces the total amount of reactive oxygen species (ROS) to alleviate oxidative damage, it also prevents the transmission of exercise signals, such as NO and H2O2 (Cobley et al., 2015; Shannon et al., 2022). The contradictory effects of exercise adaptation and the elimination of exercise-induced oxidative stress must be balanced to optimize exercise training outcomes (Booth & Thomason, 1991; Martinez-Ferran et al., 2020; Merry & Ristow, 2016).
Our research group has previously investigated the effects of pre-exercise supplementation of various antioxidants, including vitamin C and hydrogen gas (H2) in a rodent model (Chaoqun et al., 2021). We found that inhalation of H2 promotes the biogenesis of beneficial exercise adaptation while maintaining antioxidant capacity in skeletal muscle. Hydrogen gas is different from other antioxidant supplements in that it reduces perhydroxyl radicals (OH−) and peroxynitrite (ONOO−) levels (Kawamura, Higashida & Muraoka, 2020; Ohsawa et al., 2007; Ostojic, 2015), while retaining the superoxide anion level that serves as a physiological signal of the cell (Ohsawa et al., 2007; Ostojic, 2015). Ohsawa et al. (2007) have shown that hydrogen can rapidly trap and reduce these free radicals. Typically during exercise, skeletal muscle contraction induces an increase in NO production through activation of NO synthase isoforms (Millar et al., 2014; Moncada, 1997; Roque et al., 2013). The reaction of O2− with NO to produce ONOO− occurs approximately three times faster than the dismutation of O2− to produce hydrogen peroxide (Powers & Jackson, 2008). This is the key reason why ONOO− produced during exercise reduces the bioavailability of NO levels. However, at the same time, ONOO− generated during exercise decreases the level of NO (Blanco-Rivero et al., 2013). Suhr et al. (2009) reported that endothelial nitric oxide synthase (eNOS) content and activity and NO production decreased after two acute training sessions in ice hockey players. These reductions occur because under stress conditions ROS and NO combine to produce ONOO−, which exacerbates eNOS uncoupling (Rochette et al., 2013; Zweier, Chen & Druhan, 2011), and ONOO− oxidizes the cofactor tetrahydrobiopterin (BH4) (Rochette et al., 2013; Tejero, Shiva & Gladwin, 2019).
Given all these findings, we hypothesized that H2 supplementation would have an antioxidant effect that would protect NO signaling in response to exercise training. To test this hypothesis, we designed a 3-week, randomized, double-blind, controlled study with a crossover design to investigate the effect of pre-exercise inhalation of H2-rich gas on post-exercise NO signaling in a group of professional rugby players. To track changes in NO levels, we assessed levels of the NO synthesis precursor L-arginine (L-Arg) and the cofactor BH4. We further hypothesized that H2 supplementation would reduce oxidative damage and inflammation, which would result in beneficial vascular adaptation after a high-intensity exercise. To test whether H2 supplementation would reduce oxidative stress and inflammation, we measured the levels of oxidative damage markers and inflammatory factors.
Methods
Participants
A total of 24 male athletes, with mean age 23 ± 2.65 years, body weight 88.32 ± 6.53 kg and height 183.73 ± 5.88 cm, volunteered to participate in this study. The participants were high-level professional rugby players with a minimum of 6 years of professional training. All participants lived together in the athletes’ dormitory and had a similar diet during training, as provided by the athletes’ canteen. They did not take any dietary supplements. No participant had significant injury or illness during the experiment, neither consumed alcohol or beverages with a high caffeine content in the 24-h before each test, nor consumed any substances known to alter hormonal responses.
This study was approved by the Human Experimental Ethics Committee of Nanjing Sport Institute (RT-2023-10) and was carried out in line with the Declaration of Helsinki on Human Experimentation. Before recruiting participants, this study has been registered in the Chinese Clinical Trials Registry (Registration number: ChiCTR2300071589). All participants provided written informed consent before the commencement of the study.
Study setting and design
This study used a randomized, double-blind, placebo-controlled, crossover design. The minimum sample size for achieving the required statistical power was estimated with G*Power, version 3.1.9.6, as 20 participants using two-way analyses of variance (ANOVA) with repeated measures, with assumptions of type 1 error at 0.05, type 2 error at 0.20, and an effect size of 0.30. The 24 recruited participants were randomly assigned to one of two groups (received H2 supplementation first, or received placebo first), in equal numbers by a person outside the research team who did not know the researchers or participants. The randomization sequence was created using Microsoft Excel, with random block sizes of 2, 4, and 6. Hydrogen and placebo were prepared by a third person (did not participate in experiments) before each intervention. The equipment that makes hydrogen and placebo was completely blinded to the participants by custom-made boxes. Participants and researchers could only see the hoses from the equipment without knowing the content. The two gas outlet hoses were identical in shape and colour. The researchers followed a schedule list given by the research team leader to administer inhalation of hydrogen-rich air or placebo air.
During the experimental period, the coaches of the athletes constructed their training cycles as a normal training week followed by an adjustment week, with six days of training (D1–D6) and one day of rest (D7) in each week. All participants followed the same training program. The training week comprised a training program with higher intensity than that in the adjustment week. The daily training program typically included physical exercise for 90–120 min, high-intensity interval training for 30–45 min, team technique and tactics training for 90–120 min, individual technique training for 60–90 min, and recovery including stretching and physiotherapy for 60–90 min. The training program during that week before each test was controlled, and the daily training program was recorded. The training program during the third week was exactly the same as that in the first week (Appendix S1). The training program for the adjustment week was the same as for the training weeks but with a lower exercise intensity and a focus on recovery.
During the first training week, each group received the assigned supplement (H2 or placebo) for 20 min starting 1 h before training on each training day. The seventh day in each week was a rest day, with no training program scheduled and no H2 or placebo intervention given. During the second week, considered as the washout period, no participant received a supplement but participated in low-intensity training (Aoki et al., 2012; Botek et al., 2022; Jebabli et al., 2023). During the third week, each group received the supplement they had not received during the first week (crossover design) while participating in the same high-intensity training as in the first week. Blood samples were collected prior to training on D1, after training on D6, and on non-training D7, of the first and the third weeks.
H2-rich gas
Hydrogen gas can be inhaled directly into the lungs and quickly transported through the blood to the tissues (Kuropatkina et al., 2023; Ohsawa et al., 2007). It has been reported that participants could consume more hydrogen in a short period of time than if hydrogen-rich water was ingested (Huang et al., 2010; Sha et al., 2018; Valenta et al., 2022). In order not to interfere with the athletes’ training routines, we chose to administer the hydrogen supplementation by inhalation.
H2-rich gas was produced from a hydrogen-oxygen ventilator (Wuduoyun Enterprise Management Technology Co., Ltd., X9), with a known H2 and oxygen concentrations of 66.7% and 33.3%, respectively. The placebo was compressed normal air. Participants breathed the gas through a mask. The mask was linked to a mixing chamber via a hose. Neither the person administering the gas, nor the participants, were aware of which gas was given.
Blood sample collection
Blood samples were drawn by a phlebotomist from the antecubital vein. Blood was collected three times in the first week and three times in the third week, with no blood obtained during the second (rest/washout) week. The blood was drawn 1 h before training on D1 (before H2 or placebo inhalation), 1 h after training on D6, and 1 h before training on D7 (at the same time as on D1). Blood sampling is performed 1 h after exercise to assess the peak physiological responses induced by exercise (Peake et al., 2017; Shibayama et al., 2020; Tominaga et al., 2021).
Blood samples were centrifuged (3K15, Sigma) at 1,000× g for 15 min at 4 °C, and the serum was transferred to Eppendorf tubes and stored at −80 °C (Sanyo Electric Co., Ltd., Osaka City, Osaka, Japan) before analysis.
Measured blood variables
We detected L-Arg, the precursor of NO synthesis, and its auxiliary factor BH4 to track the changes in NO levels. Enzyme-linked immunosorbent assay (ELISA) kits were used to assess serum NO (BC1475; Beijing Soleibo Technology Co., Ltd., Beijing, China), eNOS (CSB-E08322h; Wuhan Huamei Biological Engineering Co., Ltd., Wuhan, Hubei, China), L-Arg (K749; BioVision), and BH4 (OKEH02612; USA Avia Biotechnology Co., Ltd., San Diego, CA, USA). The procedures were performed in strict accordance with the kit instructions.
Overload is a core training principle for athletes to improve their physical performance. Such an overload training is usually accompanied with oxidative stress and inflammation in the body (Markus et al., 2021; Powers & Jackson, 2008). The ONOO− formed by NO and O2− not only reduces the utilization of NO, but also causes DNA breakage, lipid oxidation (Carr, McCall & Frei, 2000) and protein nitration (Marletta, 2021; Powers & Jackson, 2008). Given the antioxidant and anti-inflammatory properties of hydrogen itself, we tested oxidative damage markers and inflammatory factors to determine the effects of hydrogen on oxidative damage and inflammation. The ELISA kits were used to assess serum 8-hydroxy-2′-deoxyguanosine (8-OHdG; CSB-E10140h; Wuhan Huamei Biological Engineering Co., Ltd., Wuhan, Hubei, China), carbonylation of proteins (DTJ-1-G; Suzhou Keming Biotechnology Co., Ltd., Jiangsu, China), lipid malondialdehyde (BC0025; Beijing Soleibo Technology Co., Ltd., Beijing, China), total antioxidant capacity (BC1315; Beijing Soleibo Technology Co., Ltd., Beijing, China), interleukin-6 (IL-6) (EK106; Hangzhou Lianke Biotechnology Co., Ltd., Hangzhou, China), and IL-10 (EK110; Hangzhou Lianke Biotechnology Co., Ltd., Hangzhou, China). The procedures were performed in strict accordance with the kit instructions.
Statistical analysis
All experimental data were processed using SPSS statistical software (IBM SPSS version 25 for Windows). The mean and standard deviation (SD) are presented for all variables measured. Two-way ANOVA with repeated measures was performed to determine the effect of the Supplementation (H2 and placebo) and Time (D1, D6, and D7 of each training week), and the interaction of supplementation × time. The Shapiro-Wilk test was used to assess normal distribution of the data. When a data set did not conform to a normal distribution (i.e., for BH4 and malondialdehyde), the nonparametric Mann-Whitney test was applied. Mauchly’s test of sphericity was used to determine the homogeneity of variances. If this assumption was violated, the Greenhouse-Geisser adjustment was performed. When there was a significant interaction or main effect of the two-way repeated-measures ANOVA, post-hoc analyses with Bonferroni adjustment were conducted for pairwise comparisons of the changes between the baseline and each time point.
Results
During training, two participants withdrew from the study due to injury. Data from the two groups were combined, and data from 22 participants who completed both the H2 and placebo interventions were included in the final analyses.
Production of NO
NO
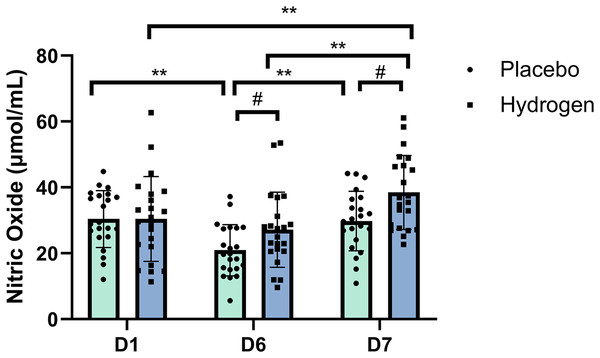
The results of the two-way repeated-measures ANOVA indicated that there was a statistically significant interaction between the main effects of supplementation and time on NO levels (F(2,41) = 6.075, p = 0.003, η2 = 0.126). A simple main effect analysis showed that Time did have a significant effect (F(2,41) = 31.798, p < 0.001, η2 = 0.431).
Group simple effect test results indicated that the NO level in response to the H2 intervention was significantly higher than that in response to placebo after exercise (D6: H2 27.15 ± 11.40 vs. placebo 20.94 ± 7.78 μmol/mL; F = 4.445, p = 0.041) and after rest (D7: H2 38.48 ± 11.24 vs. placebo 29.79 ± 9.05 μmol/mL; F = 7.963, p = 0.007).
The post-hoc comparisons indicated that, in respond to H2 supplementation, NO levels after rest were significantly higher than that at the baseline (D7, 38.48 ± 11.24 vs. D1, 30.44 ± 12.88 μmol/mL, p < 0.001) and after exercise (D7, 38.48 ± 11.24 vs. D6, 27.15 ± 11.40 μmol/mL, p < 0.001). In response to the placebo treatment, NO levels were significantly lower after exercise than at the baseline (D6, 20.94 ± 7.78 vs. D1, 30.42 ± 8.62 μmol/mL, p < 0.001) and after rest (D6, 20.94 ± 7.78 vs. D7, 29.79 ± 9.05 μmol/mL, p < 0.001). Figure 1 shows serum NO levels at different time points after receiving placebo or H2 supplementation.
Figure 1: Changes in nitric oxide levels from baseline.
Changes are after 1 week of high-intensity training with supplemental hydrogen vs. placebo given prior to exercise. **p < 0.01 for significant differences between time points in the placebo or hydrogen treatments. #p < 0.05 for significant differences between placebo and hydrogen treatments at the same time point. D1 represents baseline; D6, after 1 week of training; and D7, rest day.NOS
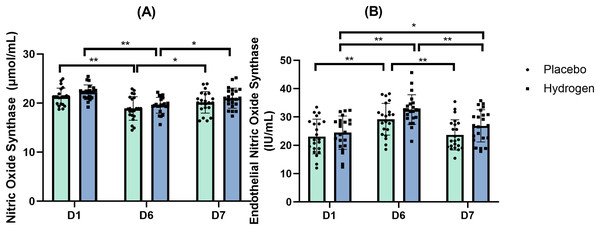
The results of the two-way repeated measures ANOVA indicated no statistically significant interaction between the main effects of Supplementation and Time on NOS levels (F(2,41) = 0.091, p = 0.913, η2 = 0.002).
Figure 2A shows the levels of NOS at different time points in response to the placebo and H2 treatments.
Figure 2: Nitric oxide synthase (A) and endothelial nitric oxide synthase (B) changes from baseline.
Changes in levels are after 1 week after high-intensity exercise training with supplemental hydrogen vs. placebo given 1 h prior to exercise. *p < 0.05 and **p < 0.01 for significant differences between time points for placebo or hydrogen treatments. D1 represents baseline; D6, after 1 week of training; and D7, rest day.Endothelial nitric oxide synthase (eNOS)
The results of a two-way repeated-measures ANOVA indicated no statistically significant interaction between the main effects of supplementation and time on the levels of eNOS (F(2,41) = 0.811, p = 0.428, η2 = 0.019). A simple main effects analysis showed that time did have a statistically significant effect (F(2,41) = 37.388, p < 0.001, η2 = 0.471).
The post-hoc comparisons indicated that, in respond to H2 supplementation, there was a significantly higher eNOS level after exercise than at baseline (D6, 32.61 ± 5.32 vs. D1, 24.47 ± 5.89 IU/mL, p < 0.001) and after rest (D6, 32.61 ± 5.32 vs. D7, 26.87 ± 5.69 IU/mL, p < 0.001). In addition, serum eNOS levels were also significantly higher than the baseline after rest (D7, D7, 26.87 ± 5.69 vs. D1, 24.47 ± 5.89 IU/mL, p = 0.039). In response to the placebo treatment, there was a significantly higher eNOS level after exercise than at the baseline (D6, 29.22 ± 5.61 vs. D1, 23.10 ± 6.13 IU/mL, p < 0.001) and after rest (D6, 29.22 ± 5.61 vs. D7, 23.70 ± 5.33 IU/mL, p < 0.001). Figure 2B shows the levels of eNOS at different time points in response to the placebo and H2 treatments.
BH4
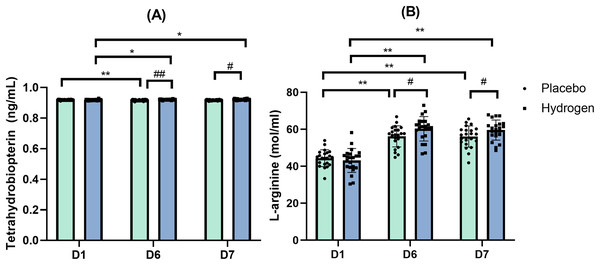
The results of Mann-Whitney tests indicated no significant differences in the levels of BH4 between H2 and placebo supplementations at the baseline on D1 (Z = −1.844, p = 0.065), but there was a significant difference after exercise on D6 (Z = −3.574, p < 0.001) and on D7 (Z = −2.679, p = 0.007).
After exercise (D6), there was a significant difference in BH4 levels between the recipients of H2 and placebo (H2: 0.922 ± 0.003 vs. placebo: 0.917 ± 0.004 ng/mL, p < 0.001). After rest (D7), there was also a significant difference in BH4 levels between H2 and placebo (H2: 0.922 ± 0.004 vs. placebo: 0.918 ± 0.004 ng/mL, p < 0.001).
In response to H2 supplementation, BH4 levels were significantly lower at the baseline than after exercise (D1, 0.919 ± 0.004 vs. D6, 0.922 ± 0.003 nmol/mL, p = 0.007), and after rest (D1, 0.919 ± 0.004 vs. D7, 0.922 ± 0.004 nmol/mL, p = 0.022). In response to the placebo treatment, the levels of BH4 were significantly lower after exercise than at the baseline (D6, 0.917 ± 0.004 vs. D1, 0.920 ± 0.002 ng/mL, p < 0.001) (Fig. 3A).
Figure 3: Tetrahydrobiopterin (A) and L-arginine (B) level changes from baseline.
Changes are 1 week after high-intensity exercise training with supplemental hydrogen or placebo given 1 h prior to exercise. *p < 0.05 and **p < 0.01 for significant differences between time points. #p < 0.05 and ##p < 0.01 for significant differences between placebo and hydrogen treatments at the same time point. D1 represents baseline; D6, after 1 week of training; and D7, rest day.L-Arg
The results of the two-way repeated-measures ANOVA indicated that there was a statistically significant interaction between the Supplementation and Time for L-Arg levels (F(2,41) = 3.383, p = 0.039, η2 = 0.075). A simple main effect analysis showed that time did have a statistically significant effect on L-Arg levels (F(2,41) = 108.822, p < 0.001, η2 = 0.722).
Simple effect test results showed that L-Arg levels were significantly higher in the H2 supplementation group than in the placebo group after exercise (H2: 60.30 ± 6.60 vs. placebo: 56.23 ± 5.70 mol/mL, p = 0.034). After rest (D7), L-Arg levels were significantly higher in response to H2 than in response to placebo (H2: 59.62 ± 5.44 vs. placebo: 56.09 ± 5.73 mol/mL, p = 0.042) (Fig. 3B).
Post-hoc comparisons indicated that in response to H2 supplementation, L-Arg levels were significantly lower at the baseline than after exercise (D6, 60.30 ± 6.60 vs. D1, 43.19 ± 6.48 mol/mL, p = 0.030) and after rest (D7, 59.62 ± 5.44 vs. D1, 43.19 ± 6.48 mol/mL, p < 0.001). Similarly, in response to placebo, L-Arg levels were significantly lower at the baseline than after exercise (D6, 56.23 ± 5.70 vs. D1, 44.40 ± 4.54 mol/mL, p < 0.001) and after rest (D7, 56.09 ± 5.73 vs. D1, 44.40 ± 4.54 mol/mL, p < 0.001).
Oxidative damage and antioxidant capacity
8-Hydroxydeoxyguanosine (8-OHdG)
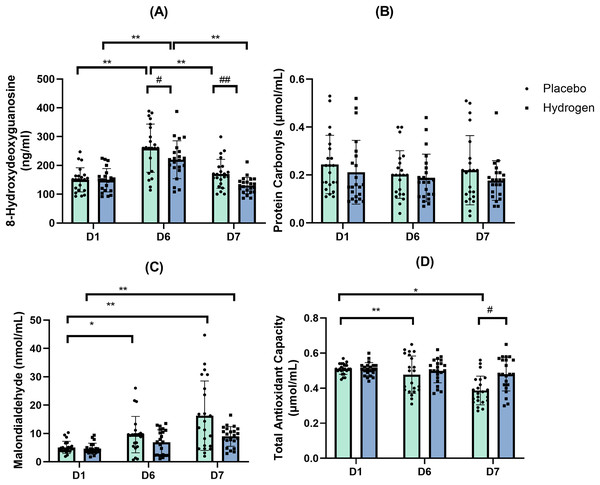
The results of the two-way repeated-measures ANOVA indicated a statistically significant interaction between the Supplementation and Time for the levels of 8-OHdG (F(2,41) = 3.906, p = 0.024, η2 = 0.085). A simple main effect analysis showed that both Supplementation (F(1,42) = 11.620, p = 0.001, η2 = 0.217) and Time (F(2,41) = 85.475, p < 0.001, η2 = 0.671) had a significant effect on the levels of 8-OHdG, a commonly used marker of oxidative stress-derived DNA damage.
The 8-OHdG levels in response to H2 supplementation were significantly lower than in response to placebo after exercise (D6) (H2: 219.77 ± 43.37.72 vs. placebo: 258.81 ± 43.23 ng/mL, p = 0.005) and rest (D7) (H2: 130.46 ± 33.39.72 vs. placebo: 170.73 ± 36.58 ng/mL, p < 0.001).
Post-hoc comparisons indicated that, in response to H2 supplementation, 8-OHdG levels were significantly higher after exercise than that at the baseline (D6, 219.77 ± 43.37.72 vs. D1, 148.65 ± 40.46 ng/mL, p < 0.001), and after rest (D6, 219.77 ± 43.37.72 vs. D7, 130.46 ± 33.39.72 ng/mL, p < 0.001). In response to the placebo treatment, 8-OHdG levels were significantly higher after exercise than that at the baseline (D6, 258.81 ± 43.23 vs. D1, 150.14 ± 41.87 mol/mL, p < 0.001) and after rest (D6, 258.81 ± 43.23 vs. D7, 170.73 ± 36.58 ng/mL, p < 0.001) (Fig. 4A).
Figure 4: Changes in levels of 8-hydroxydeoxyguanosine (A), protein carbonyls (B), malondialdehyde (C) and total antioxidant capacity (D).
Changes are from baseline after 1 week of high-intensity exercise training with supplemental hydrogen or placebo given 1 h prior to exercise. *p < 0.05 and **p < 0.01 for significant differences between time points in response to the placebo or hydrogen treatments. #p < 0.05 and ##p < 0.01 for significant differences between placebo and hydrogen treatments at the same time point. D1 represents baseline; D6, after 1 week of training; and D7, rest day.Protein carbonyls
The results of the two-way repeated-measures ANOVA indicated that there was not a statistically significant interaction between the main effects of Supplementation and Time on the levels of protein carbonyls (F(2,41) = 0.349, p = 0.707, η2 = 0.349) (Fig. 4B).
Malondialdehyde
Following placebo treatment, malondialdehyde levels at the baseline were significantly lower than that after exercise (D1, 5.07 ± 2.14 vs. D6, 9.60 ± 2.41 nmol/mL, p = 0.015) and after rest (D1, 5.07 ± 2.14 vs. D7, 16.27 ± 12.25 nmol/mL, p < 0.001). By contrast, following H2 supplementation, malondialdehyde levels after rest were significantly higher than that at the baseline (D7, 8.96 ± 3.49 vs. D1, 4.61 ± 1.94 nmol/mL, p < 0.001) (Fig. 4C)
Total antioxidant capacity
The results of the two-way repeated-measures ANOVA indicated a statistically significant interaction between the main effects of Supplementation and Time on total antioxidant capacity (F(2,41) = 5,574, p = 0.008, η2 = 0.117). A simple main effect analysis showed that both Supplementation (F(1,42) = 6.518, p = 0.014, η2 = 0.134) and Time (F(2,41) = 14.037, p < 0.001, η2 = 0.250) had a significant effect on total antioxidant capacity.
A simple effect test showed that the total antioxidant capacity in response to H2 supplementation was higher than that to the placebo at rest (H2 0.48 ± 0.10 vs. placebo 0.39 ± 0.08 μmol/mL, p = 0.001).
In response to the placebo treatment, the total antioxidant capacity was significantly lower after rest than that at the baseline (D7, 0.38 ± 0.08 vs. D1, 0.39 ± 0.08 μmol/mL, p < 0.001) and after exercise (D7, 0.38 ± 0.08 vs. D6, 0.48 ± 0.11 μmol/mL, p = 0.002) (Fig. 4D).
Inflammatory factors
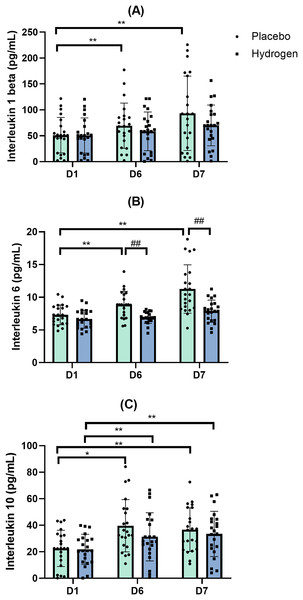
IL-1β
The results of the two-way repeated-measures ANOVA indicated no statistically significant interaction between the main effects of Supplementation and Time on the levels of IL-1β (F(2,41) = 1.616, p = 0.211, η2 = 0.037). A simple main effect analysis showed that Supplementation did not have a significant effect on IL-1β levels (F(1,42) = 1.000, p = 0.323, η2 = 0.023), whereas Time did (F(2,41) = 11.813, p < 0.001, η2 = 0.220).
In response to placebo, serum IL-1β levels were significantly lower at the baseline (D1) than after exercise (D6: 69.37 ± 43.68 vs. D1 51.20 ± 34.22 pg/mL, p = 0.022) and after rest (D7 93.30 ± 71.84 vs. D1 51.20 ± 34.22 pg/mL; p = 0.000). In response to H2 supplementation, post-exercise (D6), and post-rest (D7) values were not significantly different from D1 (Fig. 5A).
Figure 5: Changes in serum levels of IL-1β (A), IL-6 (B) and IL-10 (C) from baseline.
Changes are after 1 week of high-intensity exercise training with placebo or hydrogen supplementation given 1 h prior to exercise. *p < 0.05 and **p < 0.01 for significant differences between time points in response to placebo or hydrogen treatments. ##p < 0.01 for significant differences between placebo and hydrogen treatments at the same time point. D1 represents baseline; D6, after 1 week of training; and D7, rest day.Interleukin-6 (IL-6)
The results of the two-way repeated-measures ANOVA indicated that there was a statistically significant interaction between the main effects of Supplementation and Time on the levels of IL-6 (F(2,41) = 8.488, p = 0.001, η2 = 0.168). A simple main effect analysis showed that both Supplementation (F(1,42) = 16.762, p < 0.001, η2 = 0.285) and Time (F(2,41) = 31.662, p < 0.001, η2 = 0.430) had a significant effect on IL-6 levels.
Levels of IL-6 following H2 supplementation were significantly lower than that following placebo after exercise (D6) (H2 6.87 ± 0.87 vs. placebo 8.87 ± 2.04 pg/mL, p < 0.001) and rest (D7) (H2 7.87 ± 1.69 vs. placebo 11.26 ± 3.69 pg/mL, p < 0.001).
Post-hoc comparisons indicated that, following the placebo treatment, IL-6 levels after rest were significantly higher than that at the baseline (D7, 11.26 ± 3.69 vs. D1, 7.31 ± 1.52 pg/mL, p < 0.001) and after exercise (D7, 11.26 ± 3.69 vs. D6, 8.87 ± 2.04 pg/mL, p < 0.001) (Fig. 5B).
Interleukin-10 (IL-10)
The results of the two-way repeated-measures ANOVA indicated that there was not a statistically significant interaction between the effects of Supplementation and Time on the levels of IL-10 (F(2,41) = 1.201, p = 0.306, η2 = 0.028). A simple main effects analysis showed that supplementation did not have a statistically significant effect on IL-10 levels (F(1,42) = 1.071, p = 0.307, η2 = 0.025), whereas time (F(2,41) = 18.121, p < 0.001, η2 = 0.301) did have a statistically significant effect on IL-10 levels.
Following placebo treatment, the serum levels of IL-10 were significantly higher after exercise (D6) than that at the baseline (D1, 22.55 ± 13.65 vs. D6, 39.65 ± 19.69 pg/mL, p < 0.001) and remained higher than the baseline after rest (D7) (D1, 22.55 ± 13.65 vs. D7, 36.69 ± 16.41 pg/mL, p = 0.001). Similarly, in response to H2 supplementation, IL-10 levels were significantly higher after exercise than that at the baseline (D1, 21.82 ± 11.36 vs. D6, 31.28 ± 18.19 pg/mL, p = 0.009) and remained significantly higher than the baseline after rest (D1, 21.82 ± 11.36 vs. D7, 33.47 ± 17.06 pg/mL, p = 0.008) (Fig. 5C).
Discussion
This 3-week, randomized, double-blind, controlled, crossover study is the first, to our knowledge, to report that inhalation of H2-rich gas prior to exercise can alleviate oxidative damage and inflammation while maintaining blood NO levels. Previous studies have shown that arterial oxygen saturation decreases during exercise (Gaston et al., 2016; Goodrich, Ryan & Byrnes, 2018; Richardson et al., 1995). This hypoxia increases ROS production when oxygen availability decreases (Fuhrmann & Brune, 2017; Jiang et al., 2016; Metallo et al., 2011; Mullen et al., 2011), reducing the bioavailability of NO (Powers et al., 2020; Powers & Jackson, 2008), and is not conducive to adaptive responses to exercise and training. Previous studies have also shown that strenuous exercise reduces blood NO levels in mice (Medeiros-Lima et al., 2017; Przyborowski et al., 2018; Suhr et al., 2009), which limits the positive effects on the cardiovascular system (Gliemann, Nyberg & Hellsten, 2014). NO levels are reduced during strenuous exercise because ONOO− is formed (Marletta, 2021; Tejero, Shiva & Gladwin, 2019; Tenopoulou & Doulias, 2020). Our results showed that at the end of 1 week of exercise training (1 h after the last training session), the NO level in the blood was increased when athletes inhaled H2-rich gas before the exercise. Thus, supplementation with H2 appears to help maintain NO levels after exercise and training, protecting NO production pathways and enabling the exertion of NO’s antioxidant effect. The maintenance of an optimal level of NO in the blood has beneficial effects on blood flow that increase the availability of oxygen (and the maximum volume of oxygen uptake) and reduce the production of ROS (Heinonen et al., 2011; Oral, 2021; Stamler & Meissner, 2001; Tenopoulou & Doulias, 2020).
Increasing nitric oxide bioavailability
Most relevant studies in the literature have focused on the vasodilator effect of NO after long-term training (Millar et al., 2014), ignoring the consumption of NO by oxidative stress caused by strenuous exercise (Ashor et al., 2015; Maiorana et al., 2003). In response to the placebo treatment in our study, NO levels after exercise were significantly lower than that at the baseline. Our results demonstrate, for the first time, that the bioavailability of NO can be further increased when H2 exerts targeted antioxidant effects. Blood NO levels were higher after 1 week of H2 supplementation than at the baseline. Our results also showed that after 1 night of rest, blood NO levels of athletes exposed to H2 supplementation remained higher than after the placebo treatment. Studies have shown that NO in the blood, through cGMP dependent signaling, affects membrane fluidity and deformability, which is important for its passage through microcirculation and the delivery of oxygen to the muscles used in exercise (Kleinbongard et al., 2006). There may be two factors contributing to this result. First, during the one-week exercise, the athletes’ NO production pathway produces adaptability, and the expression of eNOS during the baseline after rest (D7) is higher than the baseline value. Second, the antioxidant effect of hydrogen targets the reduction of peroxynitrite without further damaging the level of NO.
The bioavailability of NO depends on the dynamic balance between its synthesis and consumption (e.g., by ROS). We propose that the antioxidant effect of H2 supplementation plays a role in both aspects. First, the consumption of NO and eNOS after exercise showed the same characteristics in response to placebo and H2 supplementation. It has been shown that exercise-induced increases in shear stress in the blood vessel wall increase vascular eNOS expression and activity, thereby increasing systemic NO production and bioavailability (Calvert et al., 2011; Ignarro, 1989; Napoli et al., 2004). However, this process does not appear to apply to professional athletes who train every day. In the placebo group in the present study, blood NOS levels of athletes after exercise were lower than that at the baseline, but blood eNOS levels remained the same as those at the baseline. After H2 supplementation, eNOS levels on the rest day after 6 days of exercise training were higher than those at the baseline. Thus, under stress conditions, H2 supplementation had an antioxidation effect and reduced eNOS decoupling. Secondly, we found that H2 supplementation protected the levels of BH4 and L-Arg, the key molecules in the NO synthesis pathway, and thus may help maintain NO levels after exercise. Increased ROS levels during exercise will eventually consume BH4 (Karbach et al., 2014; Li & Forstermann, 2013; Tejero, Shiva & Gladwin, 2019). Therefore, when H2 exerted its antioxidant properties, BH4 consumption was reduced. The BH4 levels of athletes after exercise were higher than their baseline levels. After rest, the formation of the eNOS dimer was not blocked, and eNOS continued to synthesize NO using L-Arg as a substrate and BH4 as a cofactor (Tejero, Shiva & Gladwin, 2019). This process protected the ability of athletes to continuously produce NO after exercise.
Reducing oxidative stress
It has been suggested that increasing NO synthesis is a double-edged sword. On one hand, it can improve blood perfusion due to its vasodilating effect. On the other hand, NO reacts rapidly with oxygen to generate ONOO−, which decreases maximal force development of the rat heart (Hong, Gokulrangan & Schoneich, 2007) and impairs the contractility of ventricular myocytes (Kraljevic et al., 2013). ONOO− can oxidize DNA bases, especially guanine, which can cause base mismatch and DNA mutation, resulting in genetic instability (Beckman & Koppenol, 1996; Carr, McCall & Frei, 2000; Kawamura, Higashida & Muraoka, 2020). These oxidation reactions destroy the structure of the protein, alter its function or render it inactive (Marletta, 2021; Wang et al., 2021). During exercise, O2− reacts with NO to produce ONOO−, which not only damages the bioavailability of NO, but also increases the level of markers of oxidative damage. Therefore, when molecular hydrogen selectively clears ONOO− (Hayashi et al., 2004), it not only protects NO signaling, but also reduces the ONOO− that causes oxidative damage to DNA in proteins. Inhalation of H2 appeared to assist in balancing these two aspects. Our results showed that blood levels of both 8-OHdG and malondialdehyde increased significantly after exercise. After rest, the malondialdehyde level was still higher than the baseline level. Supplementation with H2 did not significantly alter the change in oxidative damage markers, but reduced blood levels of 8-OHdG compared with the placebo treatment. Interestingly, in the study by Shibayama et al. (2020) in which the participants ingested hydrogen-gas after exercise (hydrogen concentration was the same as in this study), and the changes in the oxidation markers in the serum were not significant. The differences between the two reports suggest that the timing of hydrogen intake may significantly affect the level of oxidative stress. The total antioxidant capacity of athletes decreased gradually during training, and the intake of H2 enabled athletes to maintain a certain level of antioxidant capacity during high-intensity training. In addition, the total antioxidant capacity was higher in response to H2 supplementation than to placebo after one night of rest. This finding is in agreement with the results of Dobashi, Takeuchi & Koyama (2020). In their study, the participants exercised for three consecutive days, and the total antioxidant capacity was higher in the group with H2-rich water supplementation than that in the placebo group. Studies by Nogueira et al. (2021) and Nogueira et al. (2018) also showed that hydrogen increased the activity of antioxidant enzymes in the skeletal muscle of mice after acute exercise, indicating that hydrogen has a positive effect on antioxidant capacity. This finding is consistent with our results.
Relieving inflammation levels
Strenuous exercise may result in excessive production of ROS, which may lead to cell damage, with inflammation rather than adaptation. Many studies have shown that H2 has an anti-inflammatory effect (Nogueira et al., 2021, 2018; Sim et al., 2020), and our results have added further evidence in this regard. H2 supplementation resulted in a significant decrease in serum IL-6 levels both after exercise training (D6) and one day of rest (D7) compared with placebo treatment. Interestingly, in the placebo group, IL-1 levels were higher than that at the baseline both after exercise training (D6) and after rest (D7), whereas IL-1 levels in the H2 group did not show this difference. Cell production of IL-1β is a tightly regulated process that occurs only in response to inflammatory signals (Taniguchi & Sagara, 2007). During long-term exercise, the level of IL-1β increases, mediates blood vessel wall inflammation (Kawaguchi et al., 2006), increases leukocyte chemotaxis (Van Tassell, Raleigh & Abbate, 2015; Waehre et al., 2004), produces ROS, and aggravates oxidative stress (Mittal et al., 2014). In addition, inflammatory factors reduce the bioavailability of NO by stimulating the production of ROS (Peters et al., 2006). As found in the placebo group of the present study, athletes showed increased levels of inflammation during strenuous training, and the levels of inflammatory cytokines coincided with a trend toward increased markers of oxidative damage. However, H2 moderated the inflammatory response during intense training; thus, there was no longer any difference between the H2 and placebo treatments. Therefore, in the present study, the changes in inflammatory factors induced by H2 supplementation appeared to be due to anti-inflammatory effects, which would assist in protecting NO signaling.
Limitations
There are some limitations in this study. The participants were all men; thus, the conclusions obtained may not be applicable to female professional athletes. In future studies, we will ensure that the sample contains a sufficient number of women to determine whether between-sex differences exist with respect to the H2 supplementation effects.
Conclusions
The results of this study supported our hypothesis that inhaling H2-rich gas before exercise training would increase the level of NO in professional athletes after exercise and maintain the level of key molecules of NO synthesis for some time after exercise. Those effects protected the response of NO signals during exercise training. H2 played roles in both antioxidation and anti-inflammation at the same time, reducing the levels of oxidative damage markers and inflammatory factors after exercise. However, only male athletes were examined, and whether hydrogen has the same effect on female athletes needs further research.
The findings of this study provide evidence supporting the practice of H2 supplementation for reducing exercise-induced oxidative stress and injuries and facilitating recovery after training among professional athletes. Future studies are warranted to investigate the molecular mechanisms underpinning H2 supplementation effects on NO signaling in athletes during training to optimize cardiovascular adaptations.