Serum salusin levels in diarrhea- and constipation-predominant irritable bowel syndrome
- Published
- Accepted
- Received
- Academic Editor
- Vladimir Uversky
- Subject Areas
- Biochemistry, Gastroenterology and Hepatology, Internal Medicine
- Keywords
- Irritable bowel syndrome (IBS), Salusin level, Diagnosing, Prediction
- Copyright
- © 2025 Tuncel et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Serum salusin levels in diarrhea- and constipation-predominant irritable bowel syndrome. PeerJ 13:e18859 https://doi.org/10.7717/peerj.18859
Abstract
Objective
Inflammation and immunity play major roles in the etiopathogenesis of functional intestinal disorders. The salusins that were identified in this study are important because of their ease of collection, sensitivity and reliability. For that reason, the aim of this study was to analyze the importance of the levels of salusin in the blood, an inflammation-related parameter, in the diagnosis and prediction of irritable bowel disease.
Methodology
The study participants included 28 diagnosed with constipation-predominant IBS, 29 (34.1%) diagnosed with diarrhea-predominant IBS (D-IBS), and 28 (32.9%) controls. Enzyme-linked immune-sorbent assay (ELISA) method has been used for the measurement of salusin levels.
Results
Participants were 50 (58.8%) female and 35 (41.2%) male. The serum levels of salusin-α were substantially reduced in the diarrhea-predominant IBS group vs controls. There was also no major difference in the levels of salusin between the constipation-predominant-IBS and the diarrhea-predominant IBS group.
Conclusion
A major prognostic relationship was found between the level of salusins and the subgroup of D-IBS. It is well known that salusins have been related to inflammatory processes and oxidative injury in previous studies. The relationship between salusin and gastrointestinal diseases should be further investigated. Low-grade submucosal intestinal inflammation is also associated with irritable bowel syndrome. It is our belief that salusins may be useful in diagnosing, predicting or treating IBS.
Introduction
Irritable bowel syndrome (IBS) is the most widespread functional gastrointestinal disorder in the world. Its prevalence is approximately 10–20%. Middle-aged women are more likely to have this disease. The etiology of IBS is multifactorial. Diagnosis and treatment strategies are uncertain. Important pathophysiologic factors include changes in motility, intestinal hypersensitivity, increased permeability of the gut, immune stimulation, dysbiosis, inflammation, psychosocial factors, and genetic predisposition (Sperber et al., 2021; Lowell & Ford, 2012). Irritable bowel syndrome is a disorder of gut-brain interaction characterized by recurrent abdominal pain and changes in defecation or bowel habits. According to the Rome IV criteria, four subgroups have been defined: IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), IBS with mixed bowel habits (IBS-M) and unclassified IBS (IBS-U). Patients are often in need of frequent hospital visits for long-term follow-up. IBS is an important public health issue. It leads to loss of work productivity, reduced quality of life and an economic burden to the country. There is a need for biomarkers to effectively assess the prognosis of this disease given the need for prolonged follow-up. Biomarkers are important parameters used to diagnose and monitor disease. Human salusins are endogenous bioactive peptides. They share a common precursor, prosalusin. The first identification of salusins was by Shichiri et al. (2003). Salusins regulate hemodynamic balance, cytokine functions, inflammatory responses, oxidative damage, cardiovascular system modulation. They are found in many organs, involving the nervous system, endothelium, muscle, liver, lung, kidney, bone marrow, lymph nodes, spleen, small intestine, stomach and testes. Salusins are secreted by monocytes and macrophages in blood vessels. They are detectable from body fluids (blood plasma and urinate). Salusin-α and -β levels have been investigated in diseases like diabetes (DM), cardiovascular diseases, Parkinson’s, multiple sclerosis, psoriatic arthritis, rheumatoid arthritis (RA), systemic lupus erythematosus (SLE) and Behçet’s disease (Lacy et al., 2016; Shichiri et al., 2003). Though IBS is traditionally considered a functional disorder (without visible inflammation or tissue damage), subclinical inflammation has been observed in some patients with IBS, especially in the gut-associated lymphoid tissue (GALT). Salusins, being associated with inflammatory responses, may be useful as biomarkers to assess the presence or severity of low-grade inflammation that is not easily visible on routine diagnostic imaging or histological evaluations. There are also a few studies which examined the relationship between the levels of salusin and gastrointestinal disorders. In literature, it has been predicted that salusin levels would be much higher in patients with pancreatitis and can be used as a predictive indicator of disease. Based on this study, salusin levels may be related to other diseases of the gastrointestinal system. We think that salusin level, which is an inflammatory prognostic biomarker, may be altered in these patients. In our study, serum salusin levels were investigated in clinical subtypes of irritable bowel syndrome (C-IBS/D-IBS).
Methodology
Study design
Patients diagnosed with IBS according to Rome IV criteria were included in the study. The study comprised 85 participants: 28 (32.9%) with constipation-predominant IBS (C-IBS), 29 (34.1%) with diarrhea-predominant IBS (D-IBS), and 28 (32.9%) healthy controls. The exclusion criteria were pregnancy, a history of malignancy, concurrent medication use, and a history of gastrointestinal surgery. Routine laboratory tests (complete blood cell count (CBC), blood urea nitrogen (BUN), levels of liver enzymes, bilirubin, iron, vitamin B12, C-reactive protein (CRP), thyroid-stimulating hormone, and stool analysis) and colonoscopy reports were obtained from the hospital records. In addition to the routine blood tests collected from patients and healthy volunteers, a venous blood sample (5 ml) was obtained for biochemical analysis. Written informed consent was obtained from the patient and control groups.
Ethical considerations: The Ethics Committee for Health Sciences of the Medical Faculty of Celal Bayar University has approved the ethical approval. Date: 24.04.2024, Decision number: 20.478.486/2386.
Biochemical analysis
Blood samples were collected from patients and controls between 9 am and 10 am. Baseline blood sample collection were drawn in vacuum containers with Na2-EDTA (1.5 mg/ml) and centrifuged at 10 min at 3,000 RPM, the supernatant was stored frozen at −80 °C until it was examined by an expert. CBC, blood glucose values and blood lipid values for all participants were obtained from hospital records. The levels of salusin-α and salusin-β were measured by enzyme-liked immune-sorbent assay (ELISA) kit from USCN Life Science Inc. (Wuhan, China) with minimal measurable concentrations from 0.93 to 1.75 pg/mL. Sample and standard optical densities were read at 450 nm on a Spectramax ELISA analyzer (Molecular Instruments, Eagle Rock, CA, USA). Results were presented in pg/ml. All samples were studied duplicated.
Statistical analysis
Continuous values have been expressed as the mean value ± SD and median value (minimum–maximum), while categorical variables and values were reported as numbers (percentages). One-way ANOVA was used to compare normally-distributed data. Kruskal-wallis test was used for data which were non-normally distributed. Statistically significant results were defined as a p-value < 0.05.
Results
Demographic findings
There were 50 (58.8%) women and 35 (41.2%) men. Among the women, 23.5% had C IBS, 16.5% had D IBS and 18.8% were control group. Of the male participants, 9.4% had C-IBS, 17.6% had D-IBS, and 14.1% were controls. There were no major differences in gender of IBS and control groups (p = 0.202). The mean age of the participants was 54.29 ± 14.76 years. There were no major differences in age of IBS and control groups (p = 0.151). Also there were no major differences in white blood cells (WBCs) count, C-reactive protein (CRP) or hemoglobin levels of IBS and control groups.
Determination of plasma salusin-α and salusin-β levels
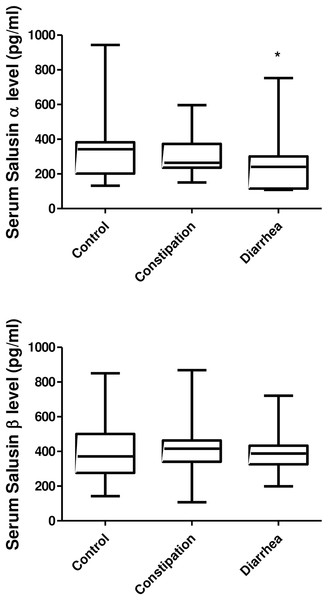
Salusin-α levels were statistically significantly reduced in the D-IBS group (p = 0.026). Salusin levels were not significant difference between C-IBS and D-IBS groups; however, the reduction in salusin-α levels was more pronounced in D-IBS. Salusin-α and salusin-β levels were not related. The number of lymphocytes was lower in the D-IBS group (p = 0.047). Demographics and laboratory characteristics are presented in Table 1. The levels of salusin-α and salusin-β are presented in Fig. 1.
| Control group | D-IBS group | C-IBS group | p-value | ||||
|---|---|---|---|---|---|---|---|
| Mean (±SD) (95% CI) | Median (min-max) | Mean (±SD) (95% CI) | Median (min-max) | Mean (±SD) (95% CI) | Median (min-max) | ||
| Age | 58.00 ± 13.89 [52.61–63.39] | 59.5 (33–80) | 50.41 ± 15.88 [44.37–56.45] | 50 (22–81) | 54.61 ± 13.89 [49.22–59.99] | 55 (20–80) | 0.151 |
| Leukocyte count (103/uL) (4.5–11) | 7,657.14 ± 2,134.102 [6,829.62–8,484.66] | 7,400 (3,800–13,000) | 8,196.55 ± 2,719.307 [7,162.18–9,230.92] |
7,200 (5,400–16,000) | 6,939.29 ± 2,153.608 [6,104.20–7,774.37] | 6,300 (4,200–12,500) | 0.091 |
| Neutrophil count (103/uL) (1.8–6.4) | 4,510.71 ± 1,532.207a,b [3,916.59–5,104.84] | 4,400 (2,300–9,000) | 4,948.28 ± 2,273.39a [4,083.52–5,813.03] | 4,200 (2,100–13,000) | 4,303.57 ± 1,875.07b [3,576.49–5,030.65] | 3,750 (1,200–9,500) | 0.464 |
| Lenfosit(103/uL) (1.2–3.6) | 2,294.29 ± 1,252.726a,b [1,808.53–2,780.04] | 1,950 (1,000–7,000) | 2,450 ± 1,173.97a [2,003.44–2,896.56] | 2,200 (900–6,000) | 1,771.43 ± 354.711b [1,633.89–1,908.97] | 1,800 (1,200–2,500) | 0.047* |
| Hemoglobin g/dl (11.5–15) | 12.40 ± 1.73 [11.73–13.07] | 12 (9–16) | 12.1 ± 1.43 [11.58–12.66] | 12.5 (9–15) | 12.7 ± 1.35 [12.24–13.29] | 12.5 (10–16) | 0.117 |
| CRP mg/dl (0–0.5) | 0.81 ± 0.93a [0.45–1.18] | 0.5 (0–4) | 1.28 ± 1.57b [0.68–1.88] | 1 (0–7) | 0.89 ± 1.27a,b [0.39–1.38] | 0.5 (0–6) | 0.117 |
| Salusin-α (pg/ml) | 343 ± 181.03a [272.80–413.20] | 342 (132–943) | 257.6 ± 158.71b [197.28–318.03] | 234 (108–752) | 307.57 ± 121.23a,b [260.56–354.58] | 264 (150–596) | 0.026* |
| Salusin-β (pg/ml) | 409.93 ± 192.13 [335.43–484.43] | 371 (142–850) | 389.48 ± 118.38 [344.45–434.51] | 382 (199–721) | 387.50 ± 148.75 [329.82–445.18] | 412.5 (107–868) | 0.954 |
Notes:
SD, Standard deviation; 95% CI, %95 confidence interval; D-IBS, diarrhea-predominant IBS; C-IBS, constipation-predominant IBS; CRP, C-reactive protein.
Figure 1: The levels of salusin alpha and beta.
Control, Control group; Constipation, Constipation-Predominant IBS group (C-IBS); Diarrhea, Diarrhea-Predominant IBS group (D-IBS); *p < 0.005.Discussion
This study investigated the variability of salusin levels, which are a marker of inflammation, in patients with IBS. It was found that salusin-α levels were decreased in the D-IBS group, suggesting its potential significance in diagnosis and prognosis. Prior research has indicated that salusins can play roles in both physiological and pathological events, particularly relating to inflammation, endothelial dysfunction, oxidative stress and cardiovascular effects. For example, IV administration of salusins to rats has been reported to induce low blood pressure and heart rate decrease (Shichiri et al., 2003; Izumiyama et al., 2005). Salusins have also been shown to be inducers of hypertrophy in rat heart muscle cells (Xiao-Hong et al., 2006; Yu et al., 2004). Moreover, it was demonstrated that patients diagnosed with hypertension, cardiovascular disease or diabetes mellitus had higher serum salusin-β levels, with these levels correlating with disease severity (Wang et al., 2020; Nazari, Minasian & Hovsepian, 2020). Contrasting effects of salusin-α and -β have been reported in some research studies, with salusin-β exhibiting proatherogenic and inflammatory effects, potentially serving as a marker of coronary heart disease (Du et al., 2013; Arkan et al., 2021). In contrast, salusin-α has been shown to have antioxidative, anti-inflammatory and antiatherogenic effects (Sun et al., 2017). Tanyeli et al. (2017) demonstrated the protective effects of salusins in an ethanol-induced experimental gastric ulcer model, showing reduced caspase-3 levels and ER stress post-treatment, suggesting its potential use in anti-ulcer therapy. A reduction in caspase-3 levels and ER stress was observed in the ulcer group after salusin treatment. Salusin-β has been found to have antiinflammatory and antioxidant characteristics in acute kidney injury (AKI) and myocardial ischemia-reperfusion injury in several studies in rats (Kimoto et al., 2010; Cakir et al., 2017). The neuro-protective effect of salusin-β in patients with a diagnosis of Parkinson’s disease has been shown in another study (Chang et al., 2018; Algul, Koc & Kaya, 2024). In addition, the relationship between oxidative stress/inflammation related diseases and salusin was also investigated (Zhao et al., 2017; Koya et al., 2012; Xu et al., 2016). Behçet’s patients have elevated salusin levels (Erden et al., 2015). In patients with psoriasis, serum salusin levels were decreased (Erden et al., 2015; Sato et al., 2010). Furthermore, the levels of salusin-β have been reported to be high in patients with systemic lupus erythematosus associated with nephritis and thrombosis (Hajialilo et al., 2023). In a study by Cakır, Ozcan & Sacmacı (2019), the levels of salusin have been shown to be related to relapsing-remitting forms of Multiple Sclerosis (MS). In another study, Albayrak, Guneyin & Celik (2024) found increased salusin-β levels in patients diagnosed with acute pancreatitis, indicating its potential as an independent prognostic biomarker for severe pancreatitis. Although IBS is generally recognised as a functional disorder (without visible inflammation or tissue damage), subclinical inflammation has been observed, particularly in the lymphoid tissue associated with the gut. Salusins are involved in immune cell modulation and regulation of inflammation. Dysregulated production of salusins can lead to an exaggerated inflammatory response in the gut. Inflammation-associated salusins may be useful as biomarkers to assess the presence or severity of low-grade inflammation. IBS has been associated with dysregulation of the autonomic nervous system, which can affect the secretion of various proteins and peptides, including salusins. Salusins are involved in vascular tone and intestinal motility. In IBS there are often changes in intestinal motility (constipation or diarrhoea). Salusins are involved in the regulation of smooth muscle contraction. Salusin-α in particular has been shown to cause vasodilatation and increase intestinal blood flow, potentially affecting intestinal motility. It is thought that salusins may play a role in modulation of the gut-brain axis. Salusin may affect brain-gut communication through its effects on the vascular system and inflamm-α atory processes. It has been suggested that salusin-α may cross the blood-brain barrier and affect central mechanisms involved in gastrointestinal regulation (Hojo & Nagahara, 2022; Sinagra et al., 2016). Zhao et al. (2023) showed that salusins may affect the activation of mast cells involved in the pathophysiology of IBS. Histamine released from mast cells may contribute to intestinal motility disorders, pain and bloating in IBS patients (Zhao et al., 2023). Chen et al. (2023) examined the interaction between salusins and the gut-brain axis in animal models with stress-induced IBS. The results showed that the increase in salusin-α expression was associated with stress-induced colonic motility disorders and pain perception. It supported that salusins may act as mediators in the gut-brain communication pathway (Chen et al., 2023). However, the direct relationship between salusins and gut microbiota is not fully known. It is possible that salusin peptides influence gut microbial composition through their effects on intestinal motility and inflammation. Despite extensive research, the exact mechanisms underlying IBS are not yet clear.
In our study, serum salusin-α and lymphocyte levels were significantly lower in the D-IBS group. Due to the relationship between salusins and inflammation, it is possible that salusin levels may vary in IBS patients. Since salusins have been shown to be associated with inflammation, oxidative stress and cell death, it suggests that altered levels of this peptide may be associated with gastrointestinal inflammation. Dysregulated salusin signalling through increased or decreased expression of these peptides may lead to various symptoms observed in IBS, including abdominal pain, bloating, altered bowel habits and visceral hypersensitivity. Few studies have investigated the association of salusin with gastrointestinal system diseases. We observed that serum salusin levels in irritable bowel syndrome subtypes (C-IBS/D-IBS) have never been studied before. It is important because it is the first study in the literature. The limitations of our study are that it was single-centre and included a small number of patients. More comprehensive studies in larger patient groups are needed.
Conclusion
In conclusion, a significant prognostic relationship was found between salusin levels and IBS subgroups (D-IBS/C-IBS). The biomarkers obtained in this study are important because they are easy to obtain, sensitive and reliable. The role of salusins in the physiopathology of gastrointestinal system-related diseases has not yet been elucidated. In order to use it as a biomarker in IBS, more studies investigating its relationship with other gastrointestinal inflammatory diseases (Crohn’s disease, ulcerative colitis, microscopic colitis) should be performed. This study may be a guide for new studies to be planned in the future. We think that salusins may be important in diagnosing, predicting or treating IBS. The potential of salusins as therapeutic targets for the management of IBS symptoms should be investigated. In addition, multicentre studies with different immunological and genetic parameters investigating the role of salusins in the pathogenesis of IBS are needed.