Risk assessment of heavy metal toxicity induced by platinum accumulation in tumor patients
- Published
- Accepted
- Received
- Academic Editor
- Viktor Brygadyrenko
- Subject Areas
- Biochemistry, Toxicology, Oncology, Pharmacology
- Keywords
- Platinum, Toxicity, Heavy metal, Chemotherapy, Risk assessment
- Copyright
- © 2025 Zhang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Risk assessment of heavy metal toxicity induced by platinum accumulation in tumor patients. PeerJ 13:e19375 https://doi.org/10.7717/peerj.19375
Abstract
Background
Many studies have focused on adverse reactions caused by platinum drugs but neglected subsequent toxicities and the mechanisms during patient recovery after chemotherapy with different platinum drugs, which need attention because of the heavy metal platinum.
Objectives
We aimed to explore the correlations between platinum accumulation, hematological indices, and clinical toxicity in patients after a metabolism period following platinum drug chemotherapy, to better understand real-world clinical toxicity caused by platinum accumulation.
Methods
We enrolled patients receiving platinum chemotherapy, specifically cisplatin, oxaliplatin, or carboplatin. On the 25th day post-chemotherapy, we measured serum platinum concentrations and hematological indices, documented clinical toxicities, and subsequently performed correlation analyses.
Results
The serum platinum concentrations in oxaliplatin-, cisplatin-, and carboplatin-treated patients were 208.60, 349.15 and 211.30 µg/L (χ2 = 51.755, p < 0.001), respectively. Mediation effect analysis showed that decreased erythrocyte, hemoglobin and glutamic-pyruvic transaminase individually mediated 21.39, 12.0 and 10.94%, respectively, of the platinum positive effect on fatigue. Decreased erythrocyte counts mediated 5.89%, while increased creatinine mediated 5.2% of the platinum positive effect on adverse reactions. The cutoff values of hematological indices, the risk of adverse reactions and fatigue were also obtained in this research which will be applied in clinical practice.
Discussion and Conclusions
Platinum accumulation, by disrupting the red blood cell system and liver and kidney function, influences fatigue severity and common adverse reactions in patients during the post-chemotherapy recovery period.
Introduction
Despite the high number of tumor-targeted drugs available, platinum-based anti-tumor drugs are still widely used as first-line drugs for many types of cancer, including of digestive tract, lung cancer and gynecological tumors, due to their effectiveness and low cost (Farrell, 2011; Gao et al., 2016; Li et al., 2019). However, the incidence of adverse reactions to platinum drugs is high, with problems including gastrointestinal reactions, rashes, and renal damage. For instance, cisplatin is consistently associated with gastrointestinal complications and nephrotoxic effects (Chopra et al., 2016), while oxaliplatin predominantly induces neurotoxicity and hypersensitivity reactions (Yamauchi et al., 2015). Additionally, carboplatin has been documented to cause peripheral neuropathy (Pujade-Lauraine et al., 2010). Moreover, several platinum drugs have been reported to have common toxicities, such as gastrointestinal reactions including diarrhea and vomiting (Lin et al., 2022). Cancer-related fatigue has also been mentioned to result from chemotherapy with platinum drugs (Izgu, Ozdemir & Basal, 2019; Modest et al., 2019).
Most of the uncomfortable symptoms mentioned above were reported during medication, but some studies have pointed out more long-term effects of platinum drugs. Platinum accumulation has been observed in patients 20 years after chemotherapy of platinum drugs (Chovanec et al., 2017). Accumulation of platinum metal induced by the use of oxaliplatin can cause neurotoxicity (Wei et al., 2021). Even 10 years after cisplatin chemotherapy, platinum levels remain positively correlated with creatinine levels, which remained significantly higher than before chemotherapy, indicating sustained damage to renal function (Boer et al., 2015), and indicative of long-term toxicity induced by platinum drugs. In our hospital, the usage of platinum drugs accounts for the highest proportion (31.68%) of all anti-tumor drugs. Patients report Michaelis lines, fatigue, nausea, vomiting and diarrhea several months after chemotherapy with cisplatin and oxaliplatin. Significant correlations between platinum accumulation and symptoms of discomfort were found in the previous research (Zhang et al., 2020). In theory, platinum drugs should be completed eliminated after 5.5 times the half-lives, but after the elimination of platinum drugs, the residual heavy metal platinum is highly likely to be similar to heavy metals such as lead, mercury, and cadmium, causing acute or chronic damage to various organs in the human body, including of anemia, asthma, immunotoxicity and nephrotoxicity (Bridges & Zalups, 2017; Orr & Bridges, 2017; Satarug et al., 2020).
Hence, in order to explore the clinical toxicities in patients after the original complete drug elimination period, we collected and analyzed blood samples from patients, recorded fatigue and adverse reactions, and then established the relationships between platinum, hematological indices and clinical toxicity, to reflect the real-world clinical toxicity caused by platinum accumulation.
Materials and Methods
Participants
All patients were diagnosed by a doctor and given a medication regimen according to the National Comprehensive Cancer Network (NCCN) guidelines. As the interval period was 5.5 times the half-life of the drugs (Zhang et al., 2021; Zhang et al., 2020), oxaliplatin has a half-life of 46 h, whereas that for carboplatin is 29 h, and cisplatin is 72 h (according to the instructions provided by the manufacturer). The chemotherapy cycle was set for more than 3 weeks usually 25 days, which is far more than 16.5 days (the 5.5 time the half -life of the drugs) according to the drug elimination and the clinical requirements of the NCCN. Based on this guideline, the 25th day after termination of chemotherapy was determined to be the appropriate time point for sample collection and physical condition assessment, since it was at the longest interval time before the next cycle. Hence, we recruited 370 patients with platinum drug chemotherapy, including 93 patients who were treated with oxaliplatin, 150 with cisplatin and 127 with carboplatin in different courses of chemotherapy, from the Cancer Hospital of Shantou University Medical College in Shantou, Guangdong Province of China, 2020 to 2022.
Inclusion and exclusion criteria. Inclusion: For these patients, physical examination was implemented before therapy and obtained the results: liver, kidney function and blood indicators are basically in the normal range, with a score of the standard from Eastern Cooperative Oncology Group (ECOG) less than one, which meant normal activity ability. The age of patients ranged from 18 to 80 years old. Exclusion: Patients had received platinum-based chemotherapy before recruitment. Metallurgical industry practitioners, pregnancy or patients taking other metal-based drugs are also excluded.
Sample and case information collection
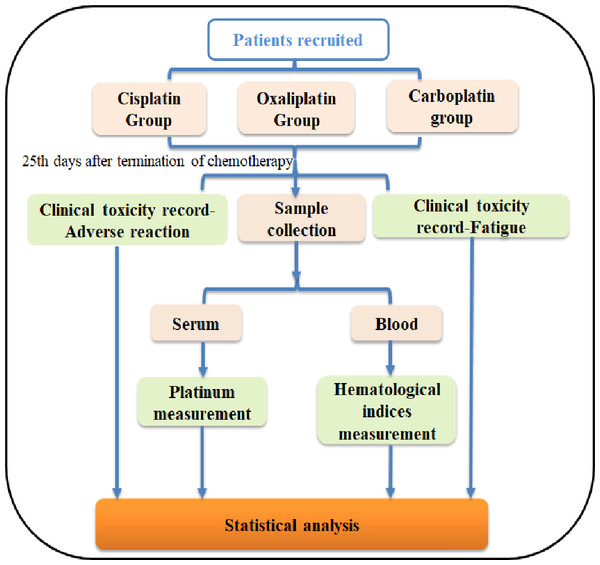
The progression of this study is illustrated in Fig. 1. A total of 4 mL of elbow venous blood was drawn from the patients, and one ml was analyzed immediately for hematological indices, while 3 ml was centrifuged (1,200 g, 3 min) to obtain serum for platinum measurement. All subjects gave their informed consent for inclusion before they participated in the study and the written informed consent has been uploaded to the journal as an attachment.
Figure 1: Flowchart of this study.
For the degree of fatigue, the Fatigue Inventory (BFI-C) was used to assess the fatigue status of follow-up patients by the clinical physician. The scale consists of nine assessment questions, with scores ranging from 0 to 10 representing the severity of fatigue.
For the adverse reactions, the clinical attending physician records the number and severity of adverse reactions that occur in patients through the Adverse Reaction Registration Form (CTCAE 5.0).
Samples and data were collected as previously described in our publications (Zhang et al., 2021; Zhang et al., 2020).
Gastrointestinal adverse reactions and rash symptoms were included in this investigation. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of the Cancer Hospital of Shantou University Medical College (2015030907).
Measurement of platinum and hematological indices
Serum and 0.5% (65%, guarantee) nitric acid (1:9) were mixed by vortexing and quantified by graphite furnace atomic absorption spectrophotometry (Jena Zeenit 650). The parameters for the AAS-650 were 265.9 nm for wavelength, 0.2 nm for slit width, 8 mA for Pt-lamp current, with an atomization temperature of 2,700 °C.
Hematological indices of samples were measured immediately after sample collection (1 mL) with the methods and instruments showed in Table 1.
| Hematological indices | Method | Instrument | |
|---|---|---|---|
| Blood cells | Leukocyte | Impedance measurement | Automatic blood analyzer (Beckman-LH780) |
| Erythrocyte | |||
| Hemoglobin | Cyanated methemoglobin colorimetric procedure | ||
| Blood biochemical index | Glutamic-pyruvic transaminase | UV | Biochemical instrument (Olympus AU5800) |
| Alkaline phosphatase | Kinetic Method | ||
| Cholinesterase | Enzymatic UV | ||
| Carbamide | Glutamate Dehydrogenas | ||
| Creatinine | Nail Creatinine | ||
| Uric acid | Enzymatic Colorimetry | ||
| Cystatin | Enzyme reagent | ||
| Phosphorus | Immunoturbidimetry | ||
Statistical analysis
Nonparametric analysis was performed, using the Kolmogorov–Smirnov test, for frequency distribution; data with skewed distributions was represented by median and 25th–75th percentile, the comparison of rates was performed by the chi-square test, skewed data was compared by non-parametric testing (Mann-Whitney U test), and correlation analysis was performed by Spearman rank correlation analysis. The concentration–response relationship was analyzed by binary logistic regression. All analyses were performed with SPSS 22.0 (IBM Corporation), GraphPad Prism 8.0 (GraphPad) software and R programming language 3.5.2. A P < 0.05 was considered as statistically significant in all analyses.
Results
Usage of platinum drugs
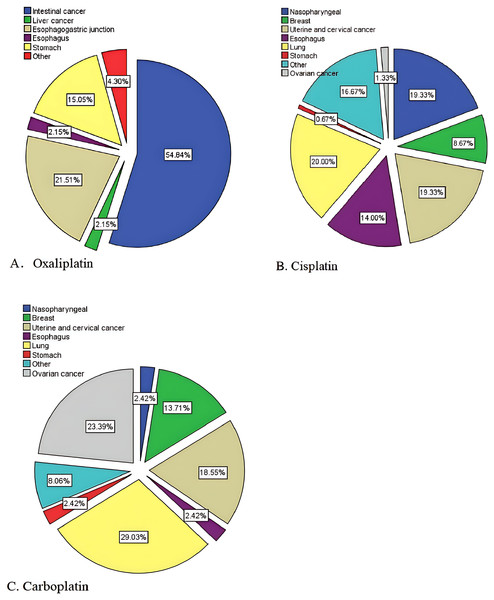
We recruited patients treated with one of three platinum drugs, oxaliplatin, cisplatin, or carboplatin, characteristics of the patients have been shown in Table 2. According to their disease information and observed that, oxaliplatin-treated patients had mostly intestinal cancer (54.8%), cisplatin-treated patients had mostly lung cancer, uterine and cervical cancer, and nasopharyngeal carcinoma (20.0, 19.3%, 19.3%), and carboplatin-treated patients had mostly lung cancer (29.0%), ovarian cancer (23.4%), uterine and cervical cancer (18.5%) (Fig. 2).
| Oxaliplatin | Cisplatin | Carboplatin | Statistic | p | |||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | n | Mean±SD or (%) | n | Mean±SD or (%) | n | Mean±SD or (%) | |||
| Age | 93 | 59.54 ± 10.68 | 150 | 55.92 ± 10.20 | 127 | 56.83 ± 10.87 | |||
| Sex | |||||||||
| Male | 67 | 72.0 | 84 | 56.0 | 32 | 25.2 | 51.45 | 0.000 | |
| Female | 26 | 28.0 | 66 | 44.0 | 95 | 74.8 | |||
| Surgery | |||||||||
| 1=No | 40 | 43.0 | 101 | 67.8 | 70 | 55.1 | 14.691 | 0.001 | |
| 2=Yes | 53 | 57.0 | 48 | 32.2 | 57 | 44.9 | |||
Figure 2: Usage distribution of the platinum drugs.
Elemental platinum accumulation resulting from platinum drug treatment
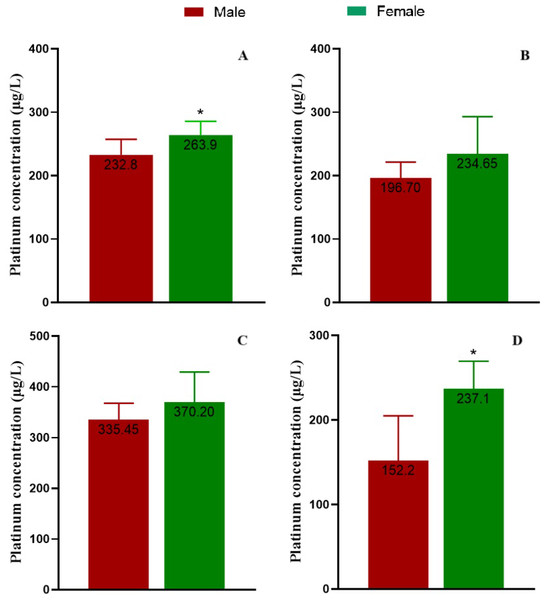
We analyzed elemental platinum in the serum of patients after the complete elimination period of chemotherapy with platinum drugs on the 25th day after the completion of certain treatment course. The serum platinum concentration of the three groups showed a non-normal distribution, so the results were analyzed using the median. The concentrations of platinum in the three groups were 208.60, 349.15 and 211.30 µg/L for oxaliplatin, cisplatin and carboplatin, respectively (Table 3). We considered the concentration difference by gender and found serum platinum concentrations tended to be higher in female patients in all groups, but the differences were significant only in the total groups combined and in the carboplatin group (p < 0.05, Fig. 3).
| Groups | Median (25th, 75th) (μg/L) | χ2 | p |
|---|---|---|---|
| Oxaliplatin | 208.60 (158.20, 267.31) | 51.755 | <0.001 |
| Cisplatin | 349.15 (237.61, 466.22) | ||
| Carboplatin | 211.30 (144.50, 294.71) |
Figure 3: Serum platinum concentrations of patients.
(A) The total participants, (B) the patients treated with oxaliplatin, (C) the patients treated with cisplatin, and (D) the patients treated with carboplatin, separately.Assessment on hematological indices of platinum accumulation
The hematological indices were measured, including leukocyte, erythrocyte counts, and hemoglobin, glutamic-pyruvic transaminase, alkaline phosphatase and cholinesterase representing liver function, carbamide, creatinine, uric acid, cystatin, and phosphorus representing kidney function, for the analysis of the relationships between anemia, kidney and liver function, and platinum accumulation. Platinum concentration positively correlated with levels of cholinesterase, creatinine, and phosphorus, but negatively correlated with erythrocyte count, hemoglobin, glutamic-pyruvic transaminase, and alkaline phosphatase (p < 0.05, Table 4).
| Hematological indices | Correlation | Partial correlation | ||
|---|---|---|---|---|
| rs | p | rs | p | |
| Leukocyte | −0.063 | 0.229 | ||
| Erythrocyte | −0.189** | 0.000 | −0.178 | 0.001 |
| Hemoglobin | −0.150** | 0.004 | −0.125 | 0.018 |
| Glutamic-pyruvic transaminase | −0.107* | 0.041 | −0.126 | 0.017 |
| Alkaline phosphatase | −0.129* | 0.014 | −0.126 | 0.017 |
| Cholinesterase | 0.132* | 0.011 | 0.116 | 0.028 |
| Carbamide | −0.020 | 0.706 | ||
| Creatinine | 0.149** | 0.004 | 0.127 | 0.016 |
| Uric acid | 0.098 | 0.062 | ||
| Cystatin | 0.031 | 0.554 | ||
| Phosphorus | 0.191** | 0.000 | 0.172 | 0.001 |
To further confirm these correlations, considering that different diseases that these patients experienced may affect blood biochemical functions, disease classification was included as a control factor for partial correlation analysis on the correlations between the platinum level and hematological indices above. Finally, we obtained negative correlations between platinum and erythrocyte count, hemoglobin, glutamic-pyruvic transaminase, and alkaline phosphatase, and positive correlations between platinum and cholinesterase, creatinine and phosphorus (p < 0.05, Table 4).
These results indicate that platinum accumulation may cause a decrease in red blood cells and hemoglobin, leading to further anemia. The incidence of anemia was 47.0%, and the risk of anemia was 1.002 times higher for every unit of increase in serum platinum levels with the 95% CI [1.001–1.003].
We established a binary logistic regression model with hematological indices as dependent variables and platinum concentration, age, gender, surgical condition, and disease type as influencing factors (dependent variables). Using this model, we predicted probabilities through pROC software (Robin et al., 2011) and calculated the optimal cutoff values for each indicator. Obtaining the following results, except for phosphorus, all other models were significant (Table 5).
| Hematological indices | Optimal cut-off | AUC | Specificity | Sensitivity | Yoden’s index | p |
|---|---|---|---|---|---|---|
| Erythrocyte count | 0.354 | 0.697 | 0.643 | 0.707 | 0.35 | <0.001 |
| Hemoglobin | 0.476 | 0.649 | 0.689 | 0.559 | 0.248 | <0.001 |
| Glutamic-pyruvic transaminase | 0.191 | 0.727 | 0.801 | 0.558 | 0.359 | <0.001 |
| Alkaline phosphatase | 0.302 | 0.633 | 0.541 | 0.725 | 0.266 | <0.001 |
| Creatinine | 0.093 | 0.737 | 0.704 | 0.708 | 0.412 | <0.001 |
| Phosphorus | 0.076 | 0.587 | 0.542 | 0.643 | 0.185 | 0.125 |
Assessment on clinical toxicity of platinum accumulation
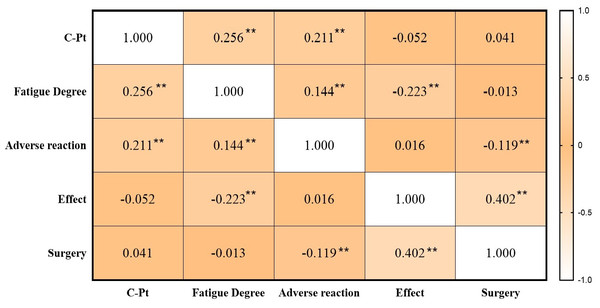
Spearman pairwise correlation analysis showed a positive correlation between platinum level and adverse reactions (including nausea, vomiting, diarrhea, and rash), and degree of fatigue. There was no significant correlation between platinum level and treatment efficacy, and surgery situation (Fig. 4).
Figure 4: Pairwise correlations between platinum, fatigue, adverse reaction, treatment effect and surgery situation.
Note: **p < 0.01.We established a binary logistic regression model with fatigue and adverse reaction as dependent variables and platinum concentration, age, gender, surgical condition, and disease type as influencing factors (dependent variables). Using this model, we predicted probabilities through pROC software (Robin et al., 2011) and calculated the optimal cutoff values for each indicator (Table 6).
| Clinical toxicities | Optimal cut-off | AUC | Specificity | Sensitivity | Yoden’s index | p |
|---|---|---|---|---|---|---|
| Fatigue | 0.693 | 0.630 | 0.606 | 0.643 | 0.249 | 0.001 |
| Adverse reaction | 0.370 | 0.648 | 0.680 | 0.566 | 0.246 | <0.001 |
Mediating factors of platinum accumulation on fatigue degree and adverse reactions
For further research of the mediation of platinum accumulation on the discomfort symptoms, mediation effect evaluation was implemented after linear regression between hematological indices, platinum fatigue and adverse reactions.
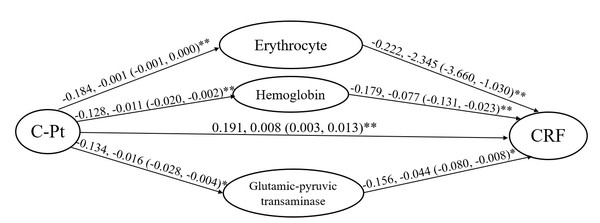
Linear regressions were applied in the mediation effect evaluation. Considering the relationships between the blood indices, platinum accumulation, adverse reactions and cancer-related fatigue analyzed above, erythrocyte count, hemoglobin and glutamic-pyruvic transaminase were contained in the fatigue model. The standardized regression coefficient (β) was obtained as a (a1, a2, a3) from the model, with the dependent variable of hematological indices (erythrocyte-a1, hemoglobin-a2 and glutamic-pyruvic transaminase-a3) and the independent variable of platinum level. The standardized regression coefficient was obtained as b (b1, b2, b3) from the model, with the dependent variable of fatigue degree and the independent variable of hematological indices (erythrocyte-b1, hemoglobin-b2 and glutamic-pyruvic transaminase-b3). The standardized regression coefficient was obtained as c from the model with the dependent variable of fatigue degree and the independent variable of platinum level. The mediation effect evaluation was calculated via the formula of a×b/c ×100% with the values obtained in the regression model. We obtained the findings that the degree of cancer-related fatigue was individually increased by 0.222- and 0.179-fold for every unit of erythrocyte and hemoglobin decrease, and 0.156-fold for every unit of glutamic-pyruvic transaminase decrease, according the “b” values. Finally, the calculation progress was implemented as below:
Platinum on fatigue via erythrocyte count: −0.184 ×−0.222/0.191=0.2139, 21.39%.
Platinum on fatigue via hemoglobin: -0.128 ×−0.179/0.191=0.1200, 12%.
Platinum on fatigue via glutamic-pyruvic transaminase: −0.134 ×−0.156/0.191=0.1094, 10.94%.
The evaluation showed that decreased erythrocytes, hemoglobin and glutamic-pyruvic transaminase separately mediated 21.39%, 12% and 10.94% of the positive effect platinum had on fatigue, according to the formula and the standardized regression coefficients. The red cell system mediated total 33.39% of the platinum effect on fatigue (Fig. 5).
Figure 5: Mediation analysis on the fatigue induced by platinum accumulation of patients included in the study.
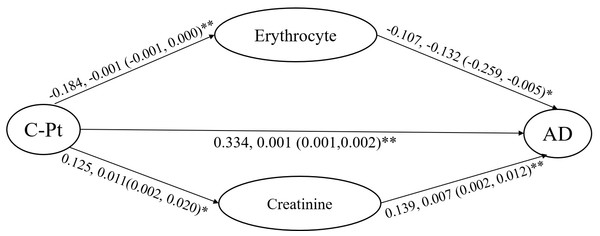
C-Pt, concentration of platinum; CRF, degree of cancer-related fatigue. The standardized regression coefficients (β) and their confidence intervals are shown.For the mediation effect evaluation of platinum-adverse reaction, erythrocyte count and creatinine were retained in consideration of their relationships with the platinum level and the degree of adverse reactions. The standardized regression coefficient (β) was obtained as a (a1, a2) from the model with the dependent variable of erythrocyte count (a1) and creatinine (a2) and independent variable of platinum level. The standardized regression coefficient was obtained as b (b1, b2) from the model with the dependent variable of adverse reaction and the independent variable of erythrocyte count (b1) and creatinine (b2). The standardized regression coefficient was obtained as c from the model with the dependent variable of adverse reaction and the independent variable of platinum level. The mediation effect evaluation was calculated via the formula of a×b/c ×100%.
The degree of adverse reaction was increased by 0.107-fold for every unit of erythrocyte decrease, and 0.139-fold for every unit of creatinine increase, according the “b” values. Finally, the calculation progress was implemented as below:
Platinum on adverse reaction via erythrocyte count: −0.107 × −0.184/0.334=0.0589, 5.89%.
Platinum on adverse reaction via creatinine: 0.139 × 0.125/0.334 = 0.052, 5.2%.
The evaluation showed that decreased erythrocyte mediated 5.89% and increased creatinine mediated 5.2% of the positive platinum effect on adverse reaction, according to the formulae and the standardized regression coefficients (Fig. 6).
Figure 6: Mediation analysis on the adverse reaction of platinum accumulation in blood of patients included in the study.
C-Pt, concentration of platinum. AD, degree of adverse reaction. The standardized regression coefficients (β) and their confidence intervals are shown.Discussion
Platinum drug application
In this research, blood and serum were obtained from patients, treated with oxaliplatin, cisplatin, and carboplatin chemotherapy, after theoretically complete drug elimination. From the distribution figure, oxaliplatin-treated patients had mostly intestinal (54.8%), cisplatin-treated patients had lung (20.0%), cervical cancers (19.3%) and nasopharyngeal carcinoma (19.3%), whereas carboplatin-treated patients had lung (29.0%), ovarian and cervical carcinoma (total 41.94%). There were significant differences in age, gender and surgery situation of patients in the three groups treated with platinum drugs, attributing to the differences in scope of use. Carboplatin is commonly used in ovarian cancer and cervical carcinoma, so that its group mostly consisted of females. The incidence of intestinal cancer in males is higher than that in females, so the group treated with oxaliplatin, which is mostly consisted of males (Sung et al., 2021).
Platinum accumulation
As the main functional substance for exerting pharmacological effects, platinum is the key element. Therefore, in clinical practice, the dosage of platinum is calculated based on body surface area to obtain a consistent initial concentration of platinum, according to the NCCN. Oxaliplatin has a half-life of 46 h, whereas carboplatin has 29 h and cisplatin 72 h. In theory, platinum drugs should be eliminated after 5.5 times the half-lives. Our previous study has reported that collections on the 25th day after therapy of platinum drugs should reflect the platinum element accumulation (Zhang et al., 2020). We found the platinum concentration in decreasing order was cisplatin, oxaliplatin and carboplatin, which is consistent with the elimination period of the original drugs. On the other hand, it also indicates that elemental platinum is not eliminated in the theoretical elimination period. Boer et al. (2015) have reported that elemental platinum continues to be present 20 years after therapy. Long term accumulation of platinum may also be caused by residual and slow release of drugs in tissues after medication (Brouwers et al., 2008). It is necessary to pay attention to platinum accumulation and to propose a treatment of heavy metal excretion in the current therapy method.
Correlations between platinum and hematological indices
This study showed negative correlations between serum platinum and erythrocyte count, hemoglobin, and as a result, platinum could increase the risk of anemia. Much research has shown the reason of anemia caused by heavy metals is due to competitive inhibition of iron by divalent metals (Hsieh et al., 2017). We found a negative effect of platinum on erythrocyte and hemoglobin levels, and further assessed the risk of anemia, which is consistent with our previous results. Results indicate that drug-induced platinum accumulation causes toxicity on the erythrocyte system, as do other heavy metals. Secondly, a positive effect was also found on creatinine and phosphorus levels, which represent nephrotoxicity. Acute and chronic heavy metal exposure has been shown to induce varying degrees of kidney disease, ranging from tubular dysfunction to life-threatening renal failure (Stepka et al., 2021). Many heavy metals have been clearly reported substances causing obvious nephrotoxicity (Sabath & Robles-Osorio, 2012). Damage caused by heavy metals during filtration through renal tubules is a major cause of nephrotoxicity. The kidney has the ability to absorb and accumulate divalent metals, and is a functional organ for metal accumulation (Safirstein, Miller & Guttenplan, 1984), about 50% of the cadmium in the body is stored in the kidney, where it can remain for more than a decade, even upon trace exposure (Gong et al., 2015; Nordberg, Goyer & Nordberg, 1975; Thevenod, 2003).
In addition, acute arsenic poisoning can lead to renal tubulointerstitial nephritis and acute tubular necrosis, characterized by proteinuria and renal papillary necrosis. Chronic or acute kidney damage caused by arsenic and cadmium is also related to oxidative stress, autophagy, or induction of cell apoptosis, and lead may cause renal tubular damage and glomerular hypertrophy (Orr & Bridges, 2017). Long-term low-dose exposure to heavy metals can also lead to interstitial nephritis (Goyer, 1997). These all indicate that heavy metals have a clear impact on the kidneys. The effect of platinum accumulation on the kidney has not been clearly reported before, but obvious nephrotoxicity has been mentioned of platinum drugs. Cisplatin may lead to long-term decreases in glomerular filtration rate (Brillet et al., 1994). The effect of carboplatin on the kidneys is manifested by a significant increase in plasma creatinine, blood urea nitrogen, and blood urea levels, with time-dependence in the observation period (Husain, Whitworth & Rybak, 2004). Oxaliplatin has also been reported to have nephrotoxicity, where there is a positive correlation between platinum and creatinine 10 years after chemotherapy, and the level of creatinine of the patients was obviously higher than before therapy, which suggests persistent damage to renal function (Boer et al., 2015). In this study, we found a significant correlation between platinum and both creatinine and phosphorus, which represent kidney function, indicating possible interference on kidney function from the accumulation of platinum.
Clinical toxicities of platinum accumulation based on hematological indices
Mediation effect evaluation showed the main cause of platinum-related fatigue was mediated by the effects on the red blood cell system (33.39%). Fatigue increases due to platinum accumulation-related decreases in erythrocyte and hemoglobin. Hemoglobin carries oxygen, and its decrease may lead to insufficient oxygen supply to the body, leading to fatigue (Aapro et al., 2017). Conversely, increasing hemoglobin intake can effectively improve a patient’s physical function (Jakel, 2002). Interestingly, we have found that platinum accumulation causes a decrease on glutamic-pyruvic transaminase, which plays a mediating role as negative effect on fatigue, finally leading to an increase in fatigue degree. It is different from our conventional understanding and has not been reported before. A few reports have indicated that fatigue is more common in women with liver cirrhosis and is related to alkaline phosphatase levels (Quarneti et al., 2015), which was not found in our study. Some researchers have also reported that platinum in the form of original forms, including oxaliplatin, cisplatin, and carboplatin, would cause liver injury (DILI) and hepatic sinusoidal obstruction syndrome (HSOS) (Bahirwani & Reddy, 2014; Qu et al., 2019; Teschke, 2022) but only focused on the drug-induced adverse reactions during medication. Several heavy metals, such as lead, cadmium, and mercury, have been reported to cause liver injury through mechanisms including oxidative stress, inflammatory response, and cellular apoptosis (Chen, Zhu & Zhou, 2023; Liu et al., 2023; Zou et al., 2020). The impact of platinum accumulation induced by chemotherapy on the liver still needs to be explored.
In addition, regression analysis showed that platinum accumulation impacts adverse reactions, such as nausea, vomiting, and diarrhea, through erythrocyte count and creatinine, manifested as a platinum accumulation-mediated decrease in erythrocyte levels, resulting in occurrence of adverse reactions. Another study has reported that anemia can lead to indigestion (Ludwig & Strasser, 2001). As we know, when kidney function is damaged, protein loss may occur, and the gastrointestinal tract may experience edema, leading to gastrointestinal dysfunction. Renal function damage is also related to an increase in intestinal pathological organisms (Anders, Andersen & Stecher, 2013). Thus, platinum accumulation may cause kidney damage, ultimately resulting in gastrointestinal symptoms, such as nausea, vomiting, and diarrhea.
This study explored the potential impact of platinum accumulation during the recovery period after chemotherapy, at a time when the elimination of platinum drugs should have been complete, from the perspective of heavy metal toxicity. The result with sampling and toxicity analysis at the specific time point can clearly distinguish from the discomfort symptoms caused by the disease itself, thus, it reflects the real-world situation of cancer patients following treatment with platinum drugs. These conclusions will lay a foundation of the mechanism of toxicity induced by platinum accumulation. Platinum accumulation is similar to other heavy metal accumulations (environmental exposure), and its long-term retention in the body may lead to discomfort symptoms, such as fatigue, nausea, and vomiting while cannot be avoided for treatment needs. However, significant correlations between platinum accumulation and treatment effect were not found in this study. It is suggested that after using platinum chemotherapy, elimination of heavy metals as an adjuvant therapy can be appropriately considered. In addition, we also established a model using R language to predict the risk of abnormal hematological indices, the risk of adverse reactions and fatigue, caused by platinum and various influencing factors, obtained corresponding cutoff values, which can be widely applied in clinical practice. Furthermore, the mediation effect analysis results obtained in this study will facilitate a deeper investigation into the molecular mechanisms underlying clinical toxicity symptoms induced by platinum accumulation, encompassing oxidative stress, ferroptosis, and other related pathways.
Conclusions
Platinum accumulation impacts fatigue degree and common adverse reactions of patients, in the recovery period after chemotherapy, by interfering with the red blood cell system, and liver and kidney function, but significant correlations between platinum accumulation and treatment effect were not found in this study.