The effects of dance intervention on clinical symptoms and cognitive deficits in hospitalized patients with chronic schizophrenia: a randomized controlled trial
- Published
- Accepted
- Received
- Academic Editor
- Marialaura Di Tella
- Subject Areas
- Clinical Trials, Cognitive Disorders, Psychiatry and Psychology
- Keywords
- Dance intervention, Aerobic exercise, Schizophrenia, Clinical symptoms, Cognitive deficits, Physiological indicator
- Copyright
- © 2025 Li et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. The effects of dance intervention on clinical symptoms and cognitive deficits in hospitalized patients with chronic schizophrenia: a randomized controlled trial. PeerJ 13:e19840 https://doi.org/10.7717/peerj.19840
Abstract
Background
Schizophrenia is a severe mental disorder that imposes significant social burdens. Traditional treatmens, however, have limited effectiveness in addressing negative symptoms and cognitive deficits. Recent studies have indicated interventions promoting sensorimotor integration may offer potential benefits for schizophrenia treatment. The current study aimes to investigate the effects of dance training as an intervention for patients with chronic schizophrenia.
Methods
This study recruited 34 individuals with schizophrenia, who were randomly allocated to either a dance intervention group (n = 18) or an aerobic exercise group (n = 14) for a 3-month program including fifty-minute sessions three times per week. Clinical symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS) and Nurses’ Observation Scale for Inpatient Evaluation (NOISE). Cognitive function was evaluated using the MATRICS Consensus Cognitive Battery (MCCB). Meanwhile, physiological indicators were also collected to explore the underlying physiological effects of the dance intervention. All measurements were taken before and after the interventions.
Results
The PANSS total scores (P < 0.01**), PANSS negative scores (p < 0.001**), NOISE score (p < 0.001**), MCCB cognitive scores, and physiological indicators were significantly changed after the intervention in both groups compared with baseline. Post-hoc analysis revealed notable improvements in the specific cognitive subscales, including the Continuous Operation Test-identical pairs (CPT-IP, p = 0.026*), Hopkins Verbal Learning Test-Revised (HVLT-R, p = 0.019*) and the physiological indicators Cystatin C (CYS-C, p = 0.037*) in the dance intervention group. Additionally, significant correlation were found between PANSS positive score and Total Bilirubin (TBIL) (r = 0.449, p = 0.028*), as well as between CPT-IP and Cystatin C (CYS-C) (r = 0.501, p = 0.008**) were found in both groups.
Conclusion
While both interventions resulted in improvements in clinical symptoms and cognitive function, the dance intervention specifically enhanced attention and verbal memory. Dance and aerobic exercise induced different changes in physiological indicators, which might be the physiological basis for improvements in clinical symptoms and cognitive function.
Introduction
Schizophrenia affects approximately 1% of the global population, placing a significant burden on the healthcare system (Sawa & Snyder, 2002). In the United States alone, the annual associated costs of schizophrenia exceed $150 billion (Cloutier et al., 2016). In China, the prevalence of schizophrenia more than doubled from 1990 to 2010 (Chan et al., 2015). However, the curative effects remain unsatisfactory worldwide (Insel, 2010). A 25-year international follow-up study found that only 37.8% of individuals with schizophrenia met the recovery criterion (Harrison et al., 2001). Schizophrenia is a complex disorder, characterized by three main domains: positive symptoms, negative symptoms, and cognitive deficits. Positive symptoms in schizophrenia, such as hallucinations, delusions, and disorganized thinking, contribute to a distorted sense of reality. Negative symptoms, on the other hand, involve the reduction or absence of normal behaviors and functions, including diminished emotional expression, lack of volition, and difficulties in performing daily activities (Jauhar, Johnstone & McKenna, 2022). Cognitive impairments in schizophrenia patients affect several domains, including attention (Arican et al., 2019), memory (Benaiges, Serra-Grabulosa & Adan, 2013) and executive functioning (Benaiges et al., 2013). These symptoms collectively impair the quality of life of affected individuals (Alptekin et al., 2005; Narvaez et al., 2008).
Antipsychotic medications are effective in managing positive symptoms, particularly during the severe psychotic episode (Tandon, Nasrallah & Keshavan, 2010). However, they are only successful for about 50% of patients and have limited efficacy for negative symptoms and cognitive decline, especially as schizophrenia progresses into the chronic stage. These medications are only successful for about 50% of patients (De Araújo et al., 2012), and have limited efficacy for negative symptoms and cognitive decline (Patel et al., 2014; Goff, Hill & Barch, 2011), especially as schizophrenia progresses into the chronic stage. A multi-site study with 1,493 participants found minimal cognitive improvement after 18 months of antipsychotic treatment, with a high discontinuation rate of 64%–82%, and the efficacy of medication diminished over time in both clinic symptoms and cognitive functions (Lieberman et al., 2005; Heinrichs, 2007). Furthermore, the efficacy of both first- and second-generation antipsychotics in addressing negative symptoms and cognitive impairments remains low (Fabrazzo et al., 2022; Wolpe et al., 2023; Nielsen et al., 2015; Feber et al., 2025). Often accompanied by various side effects such as extrapyramidal effects, restlessness, and metabolic disturbances (Leucht et al., 2009; Haddad et al., 2023; Marder & Cannon, 2019). Moreover, these side effects hindered the recovery of negative and cognitive symptoms in patients with schizophrenia. Researchers found that patients with extrapyramidal symptoms exhibited severe negative and mood-related symptoms and poorer performance on cognitive tests (Tanaka et al., 2012; Fervaha et al., 2015; Weng et al., 2019). Therefore, it is crucial to explore complementary interventions for negative symptoms and cognitive deficits in schizophrenia.
Recent research suggests that sensorimotor dysfunction in schizophrenia contributes to both clinical symptoms and neurocognitive deficits (Ford et al., 2008; Javitt & Freedman, 2015). Studies show that sensory training could enhance the integration of sensorimotor information in healthy populations (Brown et al., 2018; Sarasso et al., 2018), and may further improve higher cognitive abilities. Physical activities have been shown to improve sensory processing (Yamashiro et al., 2021; Zemková, 2022) and reduce negative (Sabe, Kaiser & Sentissi, 2020) andgeneral symptom (Takahashi et al., 2012) in schizophrenia patients. Dance, as a form of expressive art, shares the intense physical demands of musculoskeletal strength and cardiorespiratory training with sports (Schmitt et al., 2018), but also involves more complex and specialized sensorimotor processing distinct from athletic activities. It includes highly visual-auditory-motor integration (Kattenstroth et al., 2010), spatial patterns (Rehfeld et al., 2018), musical rhythm (Ono et al., 2014), emotional expression, and synchronization with external stimuli, and whole-body coordination (Brown, Martinez & Parsons, 2006). Studies demonstrated that dance therapy group led to more than 20% reduction in PANSS negative symptom score in schizophrenia patients (Ren & Xia, 2013; Xia & Grant, 2009). Additionally, a 12-week dance intervention was associated with significant improvements in cognitive function (Kong et al., 2024). This suggests that dance intervention might offer a more effective intervention than repetitive physical activities by improving the sensorimotor process, such as exercising and daily activities (Kattenstroth et al., 2010; Rehfeld et al., 2018).
Nonetheless, the impact of dance on schizophrenia lacks comprehensive investigation, necessitating additional empirical evidence. To investigate the efficacy of dance intervention and compare it with aerobic exercise, this study aims to evaluate the effects of dance intervention on clinical symptoms, cognitive function, and physiological indicators in individuals with chronic schizophrenia. Aerobic exercise will serve as an active control to delineate the specific effects attributable to dance. We hypothesize that both interventions may have positive impacts on cognitive functions and clinical symptoms. Furthermore, it is anticipated that dance intervention might result in greater cognitive improvements compared to aerobic exercise intervention in the context of chronic schizophrenia.
Materials & Methods
Participants
Sixty hospitalized patients with chronic schizophrenia were recruited from the Fourth People’s Hospital in Chengdu, China. All participants maintained a stable medication regimen for at least 8 weeks before and during the dance intervention, with no changes to their antipsychotic medication type or dosage.
Inclusion criteria were: (1) met DSM-IV criteria for schizophrenia; (2) met the chronic criteria which possess 10–30 years of disease course (Harding, Zubin & Strauss, 1992); (3) aged between 30–60 years old; (4) no history of suicide attempts within the past 6 months (Andreasen et al., 2005).
Exclusion criteria were: (1) acute schizophrenia or in the onset period; (2) brain organic disorders (3) severe chronic underlying disease symptoms; (4) overweight and underweight; (5) significant cardiovascular, neuromuscular and endocrine or other somatic disorders; (6) space claustrophobia; (7) inability to comprehend the instructions in this study.
The study was registered in the Chinese Clinical Trials Register (ChiCTR2100049273) and approved by the local ethics committee of the University of Electronic Science and Technology of China (1061420210617005). All patients were duly informed of the study’s purpose, and written informed consents were obtained from all participants. The study was performed in accordance with the 1964 Declaration of Helsinki (World Medical Association, 2013).
Study design
Participants were randomly assigned to either the dance intervention group (DI) or the aerobic exercise group (AE). The study aimed to compare the effects of dance with those of aerobic exercise, focusing on the specific impact of the dance intervention. None of the participants had prior dance experience. Clinical symptoms and cognitive function were evaluated before and after the intervention. All evaluations were performed and interpreted by the same researchers or experienced clinicians, including psychiatrists or psychiatric inpatient nurses, who were blinded to the identity of the participants and the pre- and post-test results.
Both intervention programs, DI and AE, consisted of 50-minute sessions, three times a week, over the course of three months. The programs were designed with careful consideration of the participants’ abilities and safety. Previous research has shown that mild and moderate intensity physical activity can improve cognitive performance in older adults more effectively than vigorous activity (Wu et al., 2021). Given that chronic schizophrenia patients often experience severe extrapyramidal side effects from long-term medication, which impair physical flexibility and lead to disabilities. According to a previous review of the aerobic exercise for schizophrenia patients (Stanton & Happell, 2014), we finally set the intensity of the intervention at 40%–50% of the target heart rate.
To ensure comparable exercise levels across both groups, participants were required to wear exercise bracelets to record their heart rate. The volume of exercise was measured using volume of oxygen (VO2) max, with the Karvonen formula used to calculate each participant’s target heart rate (HR). The formula, which is based on the maximum heart rate (220 minus age) (Goldberg L & Kuehl, 1988), helped ensure that all participants were exercising at a moderate intensity. The formula is as follows. (1)
The goal was for patients in the dance intervention group to achieve a target heart rate corresponding to an exercise intensity level. The target HR range for the dance group was calculated between 110 and 140 beats per minute, based on their pre-intervention HR, which was recorded and averaged. Changes in heart rate for both groups, along with the target rates, are detailed in the Supplementary File SA.
Dance intervention
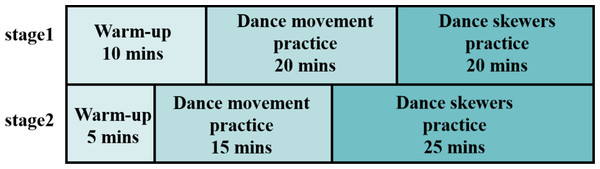
To accommodate the learning ability and the physical condition of schizophrenia patients, dance movements were taught at progressively difficult levels. The difficulty of each dance sequence was quantified (Table 1) according to a previous study on dance difficulty (Qian, 2018). This method assessed the movements across three key dimensions: time, space, and strength. Two professional dance instructors evaluated each movement on these dimensions, and scores were normalized based on the maximum value. The total difficulty score was the sum of each sub-item. As shown in Table 2, the dance sequences had three levels of difficulty, which gradually increased throughout the intervention. As the difficulty increased, so did the movement speed, the number of body parts involved, and the complexity of spatial movements.
| Dimension | Item | Calculation method |
|---|---|---|
| Time | Duration of dance sequences | The number of seconds of the duration of the dance sequence divided by 120 |
| Rate of motion | The number of beats completed in one minute divided by 200, the higher the number the faster the movement and the more difficult the dance | |
| Movements in one beat | 2 points for two movements in one beat and, 1 point for one movement in one beat, 0.5 points for one movement in two beats, and 0.25 points for four beats and one movement, and finally the cumulative score is divided by 200, with higher values reflecting faster movements and more difficult dances | |
| Motion switch times | Number of action switches in one minute divided by 40 | |
| Space | Orientation difficulty score | (1) The number of directions involving the limbs: when the limb movement involves up, down, left, right, front, back, left up, left down, right up, right down, diagonally forward, diagonally forward, diagonally down, diagonally backward, diagonally backward, etc., how many directions are involved in the scoring, and how many points are scored, and the repetition of the involved directions is not scored. (2) The number of body orientations involved: when the body movement involves the rotation or movement of the front, back, left, right, left oblique front, left oblique back, right oblique front, right oblique back, etc., the number of points will be recorded for several orientations involved, and no points will be recorded for repeated involvement. (3) Difficulty involving limb orientation: 1 point for front–back, right-back, left–right, 2 points for up-down and front–back oblique position, and 3 points for up-down oblique position. (4) Difficulty of orientation involving the body: 2 points for front–back and back, 3 points for oblique position. Difficulty of orientation = (number of limbs involved * difficulty of limbs involved + number of torso involved * difficulty of torso involved)/48 |
| Number of orientation switches | Number of switches of motion space orientation in one minute divided by 100 | |
| Number of orientation difficulty switches | The number of switches in the difficulty level of the spatial orientation of the movement in one minute divided by 40 | |
| Strength | Number of body parts involved | When a dance movement involves body parts such as fingers, wrists, arms, chest, waist, legs, knees, ankles, etc., a few points will be recorded for how many are involved, and the final score will be divided by the maximum value of 9. The higher the value, the more body parts are involved in the movement, and the higher the difficulty of the dance |
| Total score | The scores of all the entries were added together to get a difficulty score for each dance segment | |
As shown in Fig. 1, the intervention was divided into two stages. In the first stage, dance movements with strong rhythmicity were chosen, emphasizing basic movement skills. In the second stage, more complex movements were introduced, combining varied actions to train the participants’ ability to integrate complex movements. Both stages involved exercises in movement observation, imitation, music rhythm perception, spatial navigation, and body position awareness.
Throughout the intervention, emphasis was placed on the observation, perception, and imitation of dance movements. The individual’s mastery of dance movements was considered in evaluating the overall effectiveness of the training.
At the end of the intervention, two professional dance instructors assessed the participants’ progress using a four-dimensional scoring system: musical rhythm, balance, movement coordination, and movement completion. Each dimension was rated on a scale of 1 to 10, with a total score of 40. A score of 25 or higher was considered qualified. Data from participants who met this qualification standard were included in subsequent analysis steps. Selected subject assessment data are shown in Supplementary File SB.
Aerobic exercise
To ensure that the aerobic exercise intervention matched the exercise volume of the dance intervention while minimizing cognitive demands, we carefully designed the movements to be simple and safe for participants (Fig. 2). The movements in aerobic exercise: (1) came from everyday life and did not require extra cognitive learning; (2) had been used as aerobic stimulation in previous relevant studies; (3) were safe and suitable for the patients, without causing any potential injuries. We ensured that these movements matched the dance intervention in terms of exercise volume, while being simple and easy to master, without necessitating in-depth learning.
Intervention instruction
To enhance participant engagement and reduce dropout rates, we introduced dance therapy in a supportive and motivating manner. We proactively addressed patients’ concerns by highlighting the physical benefits of exercise and demonstrating the dance movements to encourage involvement. A gradual increase in movement complexity, coupled with clear instructions and positive reinforcement, ensured accessibility and sustained interest. Patient feedback, including preferences for music and teaching aids, was integrated to enhance the experience. Additionally, small rewards, such as snacks, were provided after each session, with larger incentives for those completing the full program, further bolstering motivation and enriching the overall experience. The specific description can be found in Supplementary File SC.
The formal interventions for the two groups were arranged and delivered by psychiatric inpatient nurses. Before the intervention, these nurses received a unified training course to fully master the movements, which ensured the same quality of courses delivered to the participants. Each session was staffed by two nurses: one nurse taught the dance movement based on the characteristics of the content and the patients’ comprehension ability, while the other nurse supervised the session, ensuring participants understood the instructions, corrected the movement, and prevented any accidental injuries or emergency situations according to the patients’ condition.
Measures
Clinical assessment
The Positive and Negative Syndrome Scale (PANSS), is an internationally recognized authoritative tool for measuring clinical symptoms in patients with schizophrenia, with high reliability and validity ranging from 0.83–0.99 (Kay, Fiszbein & Opler, 1987). The Positive scale (PANSS-P) mainly measures the state of schizophrenic patients in delusions, hallucinations, and some other chaotic thinking manifestations. In contrast, the negative scale (PANSS-N) mainly measure cognitive, emotional, and social deficits. The General Psychopathology Scale (PANSS-G) measures general clinical symptoms such as anxiety and tension. The subscale scores ranges from 1 to 7, with higher scores indicating more severe symptoms. The total score (PANSS-total) was the sum of the three sub-scale scores, ranging from 30–210.
| Clip | Time | Space | Strength | Total score | Difficulty degrees | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Duration | Rate of motion | Movements in one beat | Motion switch times | Orientation difficulty score | Orientation switches | Orientation difficulty switches | Body parts involved | |||
| 1 | 0.25 | 0.36 | 0.245 | 0.075 | 0.25 | 0.06 | 0.075 | 0.556 | 1.871 | low |
| 2 | 0.50 | 0.27 | 0.153 | 0.275 | 0.417 | 0.09 | 0.150 | 0.667 | 2.522 | medium |
| 3 | 0.34 | 0.51 | 0.51 | 0.175 | 0.458 | 0.16 | 0.175 | 0.667 | 2.995 | high |
Figure 1: Dance intervention program.
Figure 2: Aerobic exercise intervention program.
Clinical behavior change was assessed by the Nurses’ Observation Scale (NOISE) (Lyall, Hawley & Scott, 2004), which evaluated symptoms such as social ability, interest, personal hygiene, irritability, psychotic performance, retardation, and depression exhibited by patients over the last two weeks. All assessments were performed by experienced psychiatrists or psychiatric nurses who were blinded to the group allocation.
Cognitive function assessment
Cognitive function was assessed using the MATRICS Consensus Cognitive Battery (MCCB), a reliable tool approved by the U.S Food and Drug Administration (FDA). The Chinese version of the MCCB was developed and widely implemented in clinical settings and has good reliability and validity (Shi et al., 2015). In this study, we utilized the Chinese version of the MCCB, using computer equipment and pen-and-paper materials in a quiet and undisturbed environment. The MCCB evaluates seven key cognitive abilities related to schizophrenia through ten subtests, including processing speed (Trail Making Test, TMT; Symbol Coding, SC; Category Fluency, CF), attention/vigilance (Continuous Operation Test-identical pairs, CPT-IP), working memory (Spatial Span, SS; Digit Span Test, DS), verbal learning (Hopkins Verbal Learning Test-Revised, HVLT-R), visual learning (Brief Visuospatial Memory Test-Revised, BVMT-R), reasoning and problem-solving (Neuropsychological Assessment Battery, NAB Mazes), and social cognition (Mayer-Salovey-Caruso Emotional Intelligence Quotient Test, MEST).
Physiological indicator collection
Peripheral blood is a valuable indicator of an individual’s physiological state and is widely used in the diagnosis and monitoring of chronic diseases (Mulugeta, Suppiah & Hyppönen, 2023). Emerging evidence also suggests that certain blood metabolites are associated with cognitive function (Proitsi et al., 2018). In schizophrenia, numerous peripheral blood biomarkers have been found to deviate from normal levels, including serotonin (Tamminga, 1998), C-reactive protein (Singh & Chaudhuri, 2014), and bilirubin (Radhakrishnan et al., 2011). These abnormalities may be linked to the clinical symptoms and cognitive impairments observed in patients.
To investigate whether dance and aerobic exercise interventions can influence these physiological markers, we collected comprehensive blood data from patients with schizophrenia before and after the intervention. This included routine blood tests, as well as assessments of liver and kidney function. All clinical evaluations and blood sample collections were conducted by trained psychiatrists or experienced psychiatric nurses to ensure data reliability. Biochemical analyses were performed using a Beckman Coulter AU680 analyzer (Tran, Hoang & Greaves, 2016).
Data analysis
Data were analyzed using SPSS version 26.0 (IBMCorp., Armonk, NY, USA). Group differences in demographic variables were assessed using independent-samples t-tests and chi-square tests, including gender, age, years of education, height, weight, BMI, illness duration, age at onset, medication dosage, and number of intervention sessions. Given the small sample size, non-parametric tests were also employed to validate the results. The Mann–Whitney U test (Ruxton, 2006) was used to assess baseline differences between the dance intervention and aerobic exercise groups.
Outliers beyond three standard deviations were excluded, after which a 2 ×2 repeated-measures ANOVA was conducted to examine the clinical symptoms, cognitive functions, and physiological indicators between the two groups. To complement the repeated-measures analysis, we used generalized estimating equations (GEE) for non-parametric analysis, which are particularly useful in small-sample longitudinal studies (Hanley, 2003; Gosho et al., 2023; Tada & Sato, 2024). A Bonferroni correction was applied for multiple comparisons (Simes, 1986) to ensure the robustness of our findings.
Finally, we used partial correlation analysis to examine the relationships between clinical symptoms, cognitive function, and physiological indicators, adjusting for potential confounders including age, gender, education level, height, and weight.
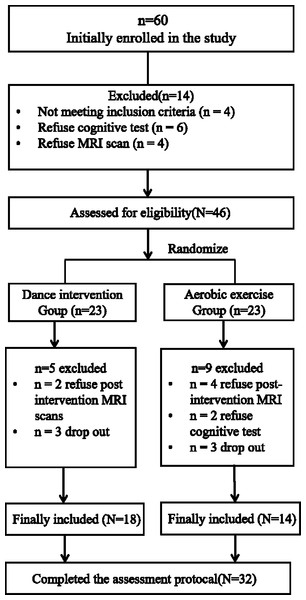
Figure 3: Participant flow in the dance intervention group and aerobic exercise group.
Results
Group comparison at baseline
Between September and December 2021, a total of twenty-eight participants dropped out during the intervention (Fig. 3). Consequently, thirty-two patients completed the full intervention program, including baseline and 3-month assessments of clinical symptoms and cognitive function. These 32 participants were included in the final analysis. There were no significant differences between dance intervention and aerobic exercise groups in terms of age, gender, education years, duration of illness, and medication dosage in chlorpromazine (CPZ) (Table 3). Furthermore, no significant differences were found in clinical symptoms or cognitive functions at baseline between dance group and aerobic exercise group (Table 4). on-parametric tests, consistent with the independent-samples t-tests, also revealed no significant baseline differences in cognitive function between the two groups (Supplementary File SC).
| Dance intervention group | Aerobic exercise group | p | |
|---|---|---|---|
| Gender (Female/ Male) | 10/8 | 5/9 | 0.49a |
| Age (years) | 50.00 (6.95) | 51.00 (8.47) | 0.90b |
| Education level (years) | 11.11 (3.25) | 10.36 (2.62) | 0.18b |
| Weight (kg) | 63.17 (9.09) | 64.93 (11.93) | 0.73b |
| Height (cm) | 162.61 (6.62) | 164.14 (6.26) | 0.79b |
| BMI (kg/cm2) | 23.79 (2.50) | 24.21 (4.90) | 0.67b |
| Duration of illness (years) | 23.44 (9.17) | 25.00 (9.91) | 0.65b |
| Onset age | 27.06 (7.68) | 26.00 (7.58) | 0.70b |
| Medication dosage in CPZ equivalents (mg) | 316.9 (142.83) | 423.1 (181.37) | 0.09b |
| Intervention times | 38.00 (7.25) | 37.36 (7.96) | 0.81b |
| Dance intervention group | Aerobic exercise group | p | |
|---|---|---|---|
| PANSS | 61.26 (7.45) | 61.20 (9.07) | 0.67 |
| NOISE | 67.15 (11.95) | 61.33 (7.32) | 0.36 |
| TMT | 21.37 (7.06) | 23.21 (6.05) | 0.88 |
| SC | 21.53 (10.80) | 18.41 (12.53) | 0.55 |
| CF | 28.00 (8.58) | 32.46 (7.54) | 0.24 |
| SS | 25.00 (12.30) | 23.77 (13.83) | 0.60 |
| DS | 29.56 (14.75) | 26.69 (11.86) | 0.92 |
| BVMT-R | 30.56 (13.32) | 25.15 (9.51) | 0.28 |
| HVLT-R | 28.53(14.97) | 35.29 (7.97) | 0.65 |
| CPT-IP | 28.18 (8.44) | 34.92 (9.94) | 0.13 |
| NAB Mazes | 23.40 (11.26) | 14.67 (15.17) | 0.15 |
| MEST | 21.93 (11.59) | 20.54 (9.32) | 0.372 |
Notes:
Indicated values are shown as mean (standard deviation).
- PANSS
-
Positive and Negative Symptom Scale
- NOISE
-
Nurses’ Observation Scale for Inpatients Evaluation
- TMT
-
Trail Making Test
- SC
-
Symbol Coding
- CF
-
Category Fluency
- CPT-IP
-
Continuous Operation Test-identical pairs
- SS
-
Spatial Span
- DS
-
Digit Span Test
- HVLT-R
-
Hopkins Word Learning Test-Revised
- BVMT-R
-
Brief Visuospatial Memory Test-Revised
- NAB Mazes
-
Neuropsychological Assessment Battery Mazes
- MEST
-
Mayer-Salovey-Caruso Emotional Intelligence Quotient Test
p values for the comparisons (two-sample t-tests) between dance group and aerobic exercise group.
Clinical symptom
As shown in Table 5, significant main effect of time main were observed for PANSS-total (p < 0.01), PANSS-N (p < 0.001), and NOISE scores (p < 0.001).
| Dance intervention group | Aerobic exercise group | Time effect p value | Group effect p value | Interaction effect p value | ||
|---|---|---|---|---|---|---|
| PANSS-P | baseline | 9.28(2.99) | 8.57(1.91) | 0.34 | 0.38 | 0.34 |
| 3 months | 9.28(3.27) | 8.29(1.98) | ||||
| PANSS-N | baseline | 21.74 (5.14) | 23.93 (5.81) | 0.001** | 0.58 | 0.58 |
| 3 months | 19.74 (5.17) | 21.27 (6.15) | ||||
| PANSS-G | baseline | 29.35(3.10) | 28.07(5.14) | 0.63 | 0.89 | 0.17 |
| 3 months | 27.41(3.43) | 29.00(5.01) | ||||
| PANSS-total | baseline | 61.26 (7.45) | 61.20 (9.07) | 0.003** | 0.93 | 0.73 |
| 3 months | 58.05 (9.50) | 58.60 (9.44) | ||||
| NOISE | baseline | 67.15 (11.95) | 61.33 (7.32) | <0.001*** | 0.14 | 0.14 |
| 3 months | 55.94 (6.41) | 53.66 (5.92) |
Notes:
Indicated values are shown as mean (standard deviation).
- PANSS
-
Positive and Negative Symptom Scale
- PANSS-P
-
the positive scores of PANSS
- PANSS-N
-
the negative scores of PANSS
- PANSS-G
-
the general scores of PANSS
- NOISE
-
Nurses’ Observation Scale for Inpatients Evaluation
After 3 months of intervention, both the dance and aerobic exercise groups showed significant reductions in PANSS total, PANSS-N, and NOISE scores, indicating improvements in the clinical symptoms of schizophrenia.
Cognitive function
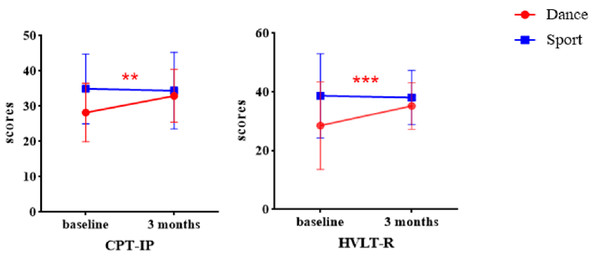
Repeated-measures ANOVA revealed significant main effect of time for TMT, CF, SS, DS, BVMT-R, and HVLT-R (Table 6). Compared with baseline, these measurements increased significantly in both groups after 3 months of intervention indicating that both dance and aerobic exercise interventions improved cognitive function in schizophrenia. Significant Time × Group interaction effects were observed in CPT-IP and HVLT-R, with a notably improvement in the dance intervention compared to aerobic exercise group (Fig. 4). In addition, the results from the GEE also showed significant interaction effect in CPT-IP and HVLT-R, further supporting the reliability of these findings (Supplemental File SD).
| Cognitive domain | Test | Dance intervention group | Aerobic exercise group | Time effect p value | Group effect p value | Time× Group interaction effect p value | |
|---|---|---|---|---|---|---|---|
| Processing speed | TMT | baseline | 21.37 (7.06) | 23.21 (6.05) | 0.003** | 0.59 | 0.62 |
| 3 months | 24.68 (8.69) | 25.57 (9.52) | |||||
| SC | baseline | 21.53 (10.80) | 18.41 (12.53) | 0.06 | 0.25 | 0.1 | |
| 3 months | 26.26 (12.94) | 18.75 (12.74) | |||||
| CF | baseline | 28.00 (8.58) | 32.46 (7.54) | 0.001** | 0.144 | 0.8 | |
| 3 months | 33.31 (7.94) | 37.38 (9.84) | |||||
| Working memory | SS | baseline | 25.00 (12.30) | 23.77 (13.83) | 0.001** | 0.88 | 0.76 |
| 3 months | 31.94 (12.60) | 31.92 (11.87) | |||||
| DS | baseline | 29.56 (14.75) | 26.69 (11.86) | 0.006** | 0.49 | 0.71 | |
| 3 months | 33.31 (11.24) | 32.77 (13.94) | |||||
| Visual learning | BVMT-R | baseline | 30.56 (13.32) | 25.15 (9.51) | 0.006** | 0.021* | 0.8 |
| 3 months | 34.31 (13.17) | 28.00 (15.65) | |||||
| Verbal learning | HVLT-R | baseline | 19.20 (15.51) | 9.34 (6.05) | 0.015* | 0.13 | 0.019* |
| 3 months | 34.00 (10.91) | 20.73 (13.47) | |||||
| Attention/Vigilance | CPT-IP | baseline | 19.41 (9.77) | 22.50 (12.31) | 0.22 | 0.98 | 0.026* |
| 3 months | 24.23 (10.03) | 21.00 (9.40) | |||||
| Reasoning and Problem-solving | MAZE | baseline | 23.40 (11.26) | 14.67 (15.17) | 0.91 | 0.266 | 0.95 |
| 3 months | 24.60 (16.48) | 15.00 (11.0) | |||||
| Social cognition | MEST | baseline | 21.93 (11.59) | 20.54 (9.32) | 0.58 | 0.37 | 0.43 |
| 3 months | 22.40 (8.49) | 17.81 (6.46) |
Notes:
Indicated values are shown as mean (standard deviation).
- TMT
-
Trail Making Test
- SC
-
Symbol Coding
- CF
-
Category Fluency
- CPT-IP
-
Continuous Operation Test-identical pairs
- SS
-
Spatial Span
- DS
-
Digit Span Test
- HVLT-R
-
Hopkins Word Learning Test-Revised
- BVMT-R
-
Brief Visuospatial Memory Test-Revised
- MAZES
-
Psychological Assessment Test Package (NAB) Maze
- MEST
-
Mayer-Salovey-Caruso Emotional Intelligence Quotient Test
Physiological indicators
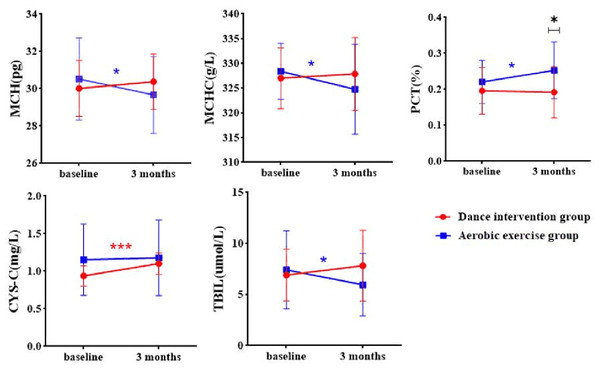
There were significant Time × Group interaction effects in mean corpuscular hemoglobin (MCH, p = 0.027), mean corpuscular hemoglobin concentration (MCHC, p = 0.048), plateletcrit (PCT, p = 0.037), cystatin C (CYS-C, p = 0.008), total bilirubin (TBIL, p = 0.041) (Table 7).
Figure 4: Group × Time interaction effect on cognitive function.
| Dance intervention group | Aerobic exercise group | Time effect p value | Group effect p value | Time × Group interaction effect p value | ||
|---|---|---|---|---|---|---|
| MCH | baseline | 30.02 (1.50) | 30.52 (2.20) | 0.35 | 0.87 | 0.027* |
| 3 months | 30.38 (1.49) | 29.67 (2.05) | ||||
| MCHC | baseline | 327.00 (6.16) | 328.38 (5.88) | 0.21 | 0.73 | 0.048* |
| 3 months | 327.87 (7.39) | 324.77 (9.09) | ||||
| PCT | baseline | 0.20 (0.07) | 0.22 (0.06) | 0.10 | 0.10 | 0.037* |
| 3 months | 0.19 (0.25) | 0.07 (0.08) | ||||
| CYS-C | baseline | 0.94 (0.14) | 1.15 (0.48) | <0.001*** | 0.17 | 0.008** |
| 3 months | 1.03 (0.14) | 1.18 (0.50) | ||||
| TBIL | baseline | 6.90 (2.54) | 7.40 (3.83) | 0.64 | 0.54 | 0.041* |
| 3 months | 7.82 (3.48) | 5.95 (3.06) |
As shown in Fig. 5, CYSC levels significantly increased in dance intervention group. MCH and MCHC significantly decreased in the aerobic exercise group, while no significant changes were observed in the dance exercise group. PCT levels significantly increased in the aerobic exercise group has a significant increase. Additional results are provided in Supplemental File SE and SF.
Correlation between clinical symptom, cognitive function and physiological indicators
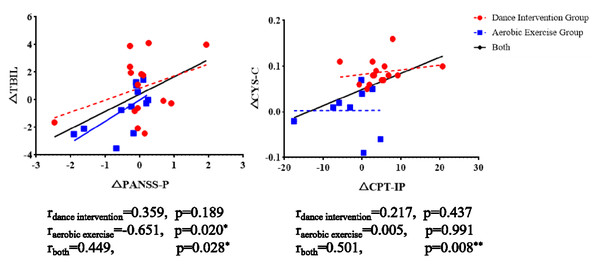
As shown in Fig. 6, a significant positive correlation was found between changes in TBIL and PANSS-P in both the aerobic exercise group and the combined group. Similarly, a significant positive association between changes in CPT-IP and CYS-C was observed in the dance intervention group and in the total sample.
Figure 5: Group × time interaction effect on physiological indicators.
MCH, Mean corpuscular hemoglobin; MCHC, Mean corpuscular hemoglobin concentration; PCT, Plateletcrit; CYSC, Cystatin C; TBIL, total bilirubin; ∗p < 0.05, ∗∗p < 0.01 and ∗∗∗P < 0.001.Figure 6: The correlation between clinical symptoms, cognitive performances, and physiological indicators.
Discussion
In this study, we found that both dance intervention and aerobic exercise improved clinical symptoms and cognitive function in chronic schizophrenic patients. Notably, only the dance intervention specifically enhanced verbal memory and attention. Additionally, several physiological indicators, including CYS-C, TBIL, MCH, MCHC, and PCT, showed significant changes after the interventions. These results suggest that dance and aerobic exercise operate through distinct mechanisms to improve clinical symptoms, cognitive functions, and physiological states in chronic schizophrenia patients. The observed improvements may reflect positive brain plasticity induced by physical activities, with dance, in particular, offering more profound effects due to its multidimensional and multisensory stimulation.
Both interventions induced beneficial effects on clinical symptoms and cognitive functions
Both interventions resulted in significant improvements in negative symptoms, information processing speed, working memory, short-term memory, and visual memory in schizophrenia. These improvements may be attributed to the shared elements of physical activity in both dance and aerobic exercise.
Dance and aerobic exercise involve whole-body movements that can enhance brain plasticity and offer potential therapeutic for individuals with schizophrenia. Previous studies have shown that interventions significantly increased hippocampal volume, a brain area associated with memory functions in older adults (Pajonk et al (2010)) and chronic schizophrenia patients (Erickson et al., 2011). Furthermore, both interventions are known to elevate levels of brain-derived neurotrophic factor (BDNF), a protein critical for neuroplasticity and brain adaptation (Numakawa, Odaka & Adachi, 2017; Nieto, Kukuljan & Silva, 2013; Lee et al., 2001). Reduced levels of BDNF are frequently reported in chronic schizophrenia patients and are associated with impairments in negative symptoms and verbal working memory (Toyooka et al., 2002; Niitsu et al., 2011). Both dance intervention and aerobic exercise were found to increase serum BDNF levels (Kim et al., 2018; Griffin et al., 2011), suggesting that the elevation of BDNF may represent shared underlying mechanism. In addition, both interventions require sustained musculoskeletal exertion and provides strength to cardiorespiratory system (Schmitt et al., 2018). Several studies have demonstrated that both the two intervention enhanced cardiorespiratory fitness in schizophrenia patients (Cheng, Sun & Yeh, 2017; Kimhy et al., 2014; Scheewe et al., 2013), which has been linked to improvement in positive (Wang et al., 2018), negative (Bryl et al., 2022), and general symptoms (Gökcen et al., 2020) as well as cognitive functions (Chen et al., 2016; Firth et al., 2017; Maurus et al., 2023).
Dance intervention induced specific cognitive improvement
Only the dance intervention led to significant improvements in attention and verbal memory. This suggests that dance may uniquely enhance the integration of sensory and motor information. Sensorimotor deficits are considered to be a core feature of schizophrenia and a key contributor to cognitive dysfunction (Javitt & Freedman, 2015; Kaufmann et al., 2015). Unlike general aerobic exercise, dance requires higher levels of sensorimotor integration and attention load. Performing dance movement requires sustained attention to auditory cues and instant synchronization of auditory, visual, and motor sensory information (Năstase, 2012). Previous studies have shown that dance improves attention switching and cognitive performance (Kattenstroth et al., 2010; Coubard et al., 2011), indicating that dance intervention may strengthen sensory-motor function of patients, and further enhance their attention control.
Additionally, the observed improvement in verbal memory may be linked to the semantic processing inherent in dance. Dance involves both action semantic (Van Elk, Van Schie & Bekkering, 2014) and music engagement (Gustavson et al., 2021), both of which rely on multisensory integration. A dance phrase typically consists of a coherent sequence of movements with clear beginnings and endings, forming a narrative structure (Grosbras, Beaton & Eickhoff, 2012). Comprehending this sequence draws on neural systems involved in processing meaning, reinforcing the connection between movement comprehension and language understanding (Bachrach, Jola & Pallier, 2016). Moreover, the musical component of dance also plays a linguistic role. Music engagement has been shown to support verbal memory through mechanisms similar to language processing (Jakobson et al., 2008; Ho, Cheung & Chan, 2003; Chan, Ho & Cheung, 1998; Jakobson, Cuddy & Kilgour, 2003; Brandler & Rammsayer, 2003). Verbal memory plays a critical role in the comprehension of linguistic texts and understanding the intentions behind spoken words in everyday life (Yıldız, Oğuzhanoğlu & Topak, 2023), which may explain the dance intervention’s positive effect on verbal memory in schizophrenia patients.
Taken together, the complexity and multimodal nature of dance may provide a unique platform for integrating primary sensorimotor information, particularly in patients with schizophrenia, thereby enhancing the brain’s capacity to process higher cognitive functions.
The two interventions have discrepant effects on the physiology level
In the dance intervention group, CYS-C levels significantly increased and were positively correlated with improved attention, as measured by CPT-IP. CYS-C is an endogenous cysteine proteinase inhibitor found abundantly in cerebrospinal fluid (Mussap & Plebani, 2004). It has been associated with neuroprotection and cognitive outcomes (Cao et al., 2022) in disorders such as Alzheimer’s disease (Sundelöf et al., 2008). Lower levels of CYS-C have also been observed in schizophrenia (Mulugeta, Suppiah & Hyppönen, 2023; Owen, Sawa & Mortensen, 2016). The post-intervention increase in CYS-C may reflect improved neural protection and cognitive function in patients receiving dance therapy.
In the aerobic exercise group, a decrease in total bilirubin (TBIL) was observed. Bilirubin, an antioxidant, is implicated in the pathophysiology of schizophrenia (Radhakrishnan et al., 2011; Yao, Reddy & Nan Kammen, 2000; Widschwendter et al., 2016; Hankøet al., 2005). Although low bilirubin levels can reduce oxidative stress, excessive reductions may diminish antioxidant capacity (Wang et al., 2023). We observed a positive correlation between TBIL levels and positive symptoms, suggesting that lower TBIL was associated with symptom reduction, possibly through improved oxidative balance. Additionally, MCH and MCHC levels decreased in the aerobic group, while PCT increased. MCH is linked to treatment-resistant schizophrenia (Cheng et al., 2024), and MCHC is associated with red blood cell aggregation and metabolic syndrome (Barshtein et al., 2004; Toker et al., 2005). Elevated PCT levels reflect increased platelet activity and inflammation (Yu et al., 2020). These findings suggest that aerobic exercise influences peripheral physiological systems, potentially modulating inflammatory or metabolic pathways relevant to schizophrenia.
In summary, the divergent patterns of physiological change between groups may reflect different underlying biochemical mechanisms triggered by dance and aerobic exercise.
Limitation
This study has several limitations. First, a relatively high attrition rate reduced the final sample size. Future studies should aim to recruit larger samples and adopt strategies to minimize dropout. Second, although the aerobic exercise group served as an active control, the absence of a non-intervention (blank) control group limits the ability to isolate the specific effects of dance. Third, fluctuations in physiological markers may have been influenced by seasonal changes or dietary variations, which were not controlled for. Additionally, premorbid variables such as substance use (Prat et al., 2021) and history of suicide attempts (Le et al., 2024), both of which can influence cognition, were not accounted for. Moreover, given the distinct influence of circadian rhythms on individuals with schizophrenia (Adan et al., 2024), future studies should adopt more rigorous controls over the timing of interventions. Finally, the short duration of the intervention precludes conclusions about long-term effects, which future longitudinal studies should address.
Conclusions
Despite these limitations, our findings demonstrate that both dance and aerobic exercise interventions led to improvements in clinical symptoms and cognitive function among patients with chronic schizophrenia. Notably, the dance intervention, characterized by its emphasis on sensorimotor integration, was associated with specific cognitive benefits, particularly in attention and verbal memory. Moreover, both interventions induced distinct physiological changes, which may partly explain the observed improvements in symptoms and cognition.
Outlook
This study supports the potential of dance as a practical, cost-effective adjunctive therapy for schizophrenia. We developed a structured, feasible dance program designed for implementation by healthcare providers, such as psychiatrists and psychiatric nurses, without requiring professional dancers. Its simplicity and standardization enhance its clinical utility.
Nevertheless, individualized approaches are essential. In some patients, high exercise intensity may exacerbate extrapyramidal symptoms. Tailoring intervention intensity to the individual’s physical and psychiatric condition is critical to maximize the benefit.
Future research should emphasize interdisciplinary collaboration, integrating neuroscience, psychology, and rehabilitation medicine. Special attention should be given to identifying the neural mechanisms by which dance influences cognition—particularly the modulation of sensorimotor integration in schizophrenia.
Supplemental Information
Demographic information
Basic details about all participants included in this study, with Group 1 representing the dance intervention group and Group 2 representing the aerobic exercise group.
Physiological indicators
Information from both before and after the intervention.
MCCB information
Participants’ cognitive functions from both before and after the intervention.
PANSS information
Clinical information from both before and after the intervention.