Relationship between heart rate variability parameters and inflammatory activity in patients with Crohn’s disease: a retrospective study
- Published
- Accepted
- Received
- Academic Editor
- Fanglin Guan
- Subject Areas
- Cardiology, Gastroenterology and Hepatology, Translational Medicine
- Keywords
- Crohn’s disease, Heart rate variability, Crohn’s disease activity index, Autonomic nervous system
- Copyright
- © 2025 Wang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Relationship between heart rate variability parameters and inflammatory activity in patients with Crohn’s disease: a retrospective study. PeerJ 13:e19893 https://doi.org/10.7717/peerj.19893
Abstract
Background
It is widely recognized that individuals with Crohn’s disease (CD) often experience impaired autonomic nervous system (ANS) function. However, the specific relationship between autonomic activity and inflammatory processes in CD remains uncertain. The objective of this study was to investigate the association between autonomic nervous function, as assessed through heart rate variability (HRV) parameters, and the Crohn’s disease activity index (CDAI).
Methods
This cross-sectional study involved 82 CD patients. Demographic and medical history data were gathered, and disease activity was evaluated using the CDAI. Short-term HRV measurements were obtained from all participants.
Results
Various adjustments to the model and trend analysis indicate that the HRV parameters are significantly negatively correlated with CDAI. After controlling for potential confounding factors, the smooth curve fitting shows that high-frequency (HF) power is negatively linearly correlated with CDAI. As the HF value increases, CDAI shows a decreasing trend. In contrast, total power (TP), low-frequency power (LF), and standard deviation of the R-R interval (SDNN) exhibit a nonlinear relationship with CDAI. To the left of the inflection point, as the values of TP, LF, and SDNN increase, CDAI shows a decreasing trend. However, to the right of the inflection point, there is no relationship between TP, LF, SDNN values, and CDAI.
Conclusion
In patients with CD, autonomic dysfunction correlates with active inflammation. Among the HRV parameters, HF, which reflects vagal-mediated activity, displayed the strongest and most consistent negative correlation with inflammatory activity.
Introduction
Crohn’s disease (CD) is a chronic, transmural inflammation involving the entire digestive tract. The cause of CD includes many factors, such as genetic or environmental factors, intestinal ecological imbalance, and mucosal immune response, but its specific pathogenic mechanism is unclear (Dolinger, Torres & Vermeire, 2024).
It is believed that the autonomic nervous system (ANS) plays an important role in the regulation of inflammatory reflex (Tracey, 2002). Heart rate variability (HRV) serves as a tool for assessing autonomic nervous system (ANS) activity. HRV parameters are categorized into those that predominantly represent parasympathetic nervous system (PNS) or vagal-mediated activity (referred to as vagal nerve-mediated HRV parameters) and those that reflect the combined influence of both the sympathetic nervous system (SNS) and the PNS (Berntson et al., 1997; Kamath & Fallen, 1993). The theory of the cholinergic anti-inflammatory pathway of the vagus nerve has led to the belief that higher HRV parameter values, especially those mediated by the vagus nerve, are related to lower levels of inflammation (Young et al., 2014; Pellissier et al., 2014; Schäfer et al., 2015).
Early studies have generally indicated that CD patients have decreased autonomic nervous function (Sharma et al., 2009; Kim, Yao & Ju, 2020). Aghdasi-Bornaun et al. (2018) reported the vagal activity was inhibited, while sympathetic nerves were dominant in CD. Engel, Ben-Horin & Beer-Gabel (2015) found that the low frequency power (LF), which mainly represents the HRV index of sympathetic activity, was negatively correlated with serum C-reactive protein (CRP), a marker associated with inflammatory activity in CD patients. Sahn et al. (2023) observed that, compared with healthy peers of the same age, baseline values of HRV parameters representing vagal tone in children with inflammatory bowel disease (IBD) were lower. Yerushalmy-Feler et al. (2022) found that in children with IBD who were in remission, the square root of the mean squared differences of successive R-R intervals (RMSSD) was notably higher compared to children experiencing active disease. Hirten et al. (2025) collected HRV parameters of CD patients via wearable devices and found no difference in the circadian pattern of SDNN during symptomatic flares vs. remission. The connection between HRV parameters and inflammatory activity in CD remains debated. Consequently, this study aimed to assess the association between ANS functioning and CD activity, as reflected by HRV parameters. If the vagus nerve is indeed involved in CD pathogenesis, HRV parameters mediated by the vagus nerve should reflect this activity.
Materials and Methods
Study design and study population
This was a retrospective study, and was approved by Research Ethics Committee of Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences (NO.GDREC2015048H, Date: Jan 22, 2015). All patients with CD treated at our hospital from February 1, 2015 to February 1, 2020 were included. Patient demographic information, personal history, medical history including age, scope of CD involvement, disease behavior, course of disease, history of surgery, history of drug treatment, and the results of laboratory tests was collected and reviewed. The diagnosis of Crohn’s disease is based on the standards recommended by the World Health Organization (WHO). The extent of CD was classified using the Montreal classification. The Crohn’s disease activity index (CDAI) was calculated to evaluate the degree of active inflammation.
Written informed consent was obtained from all participants. The following exclusion criteria were applied: (1) Absence of medical history and/or examination records; (2) Diagnosis of heart conditions such as congenital heart defects, valvular dysfunction, conduction abnormalities, ischemic heart disease, or any form of heart failure; (3) Presence of endocrine disorders, including thyroid dysfunction, diabetes, neuropathies, or other systemic inflammatory conditions; (4) Use of medications that influence the cardiovascular system and nerve conduction, such as antidepressants, analgesics, or glucocorticoids; (5) A history of smoking or alcohol consumption; (6) Pregnancy.
Measurement of HRV
HRV measurements were performed by the same well-trained doctor. The examination was carried out in a fixed temperature and light controlled environment to avoid interference, and all examinations were performed within a same time period (10:00 am to 1:00 pm). Participants were instructed to avoid caffeine, strong tea, nicotine or other stimulants for 48 h before the examination, and to have adequate sleep the night before the examination. Patients were also instructed to fast for at least 2 h before the examination, and rest for at least 15 min before the examination.
A short-range HRV analyzer (DLP6000; General Meditech Inc., Shenzhen, Guangdong, China) was used to record and evaluate patient sitting ECG signals, and an electrocardiogram was continuously recorded for 5 min using three leads of the standard limb lead II. During the examination, patients were required to be completely relaxed, breathe regularly, and to not talk or move. Technicians carefully observed the patients to ensure there was no subjective discomfort or motion artifacts during the recording. The device’s software (version 1.0.5649.19505) processed the data, and heart rate variability (HRV) was evaluated using both time-domain and frequency-domain approaches. Autonomic nervous disorders (vagus nerve or sympathetic nerve) related to specific diseases can be reflected in HRV indexes. Time-domain and frequency-domain HRV parameters calculated in this study are shown in Table 1.
| Methods | Description |
|---|---|
| Time domain indices | |
| Standard deviation of the R–R intervals (SDNN ms) | Reflects the influence of parasympathetic and sympathetic system on heart-rate variability |
| Frequency domain indices | |
| Total power (TP ms2) (0–0.4 Hz) | Reflects total variance of R–R interval over the 5 min segment |
| Low frequency power (LF ms2) (0.04–0.15 Hz) | Reflects both sympathetic and parasympathetic activity |
| High frequency power (HF ms2) (0.15–0.4 Hz) | Reflects the centrally mediated parasympathetic activity |
Statistical analysis
Continuous variables that followed a normal distribution were presented as means ± standard deviations (SD). For those that were not normally distributed, data were expressed as medians with interquartile ranges, while categorical variables were displayed as frequencies and percentages. To compare differences between groups, one-way ANOVA was employed for normally distributed variables, the Kruskal-Wallis test for non-normally distributed variables, and the chi-square test for categorical variables. Univariate linear regression analysis was conducted to examine the relationships between HRV parameters and the CDAI. In accordance with the STROBE guidelines, we reported results from unadjusted, minimally adjusted, and fully adjusted models. Covariates, once incorporated into the model, resulted in at least a 10% change in the matched odds ratio and were thus adjusted (Kernan et al., 2000). Furthermore, the generalized additive model (GAM) was applied to capture potential non-linear relationships. If a non-linear correlation was identified, a two-piecewise linear regression model was utilized to assess the threshold effects of HRV parameters on CDAI, following a smooth curve. When a clear relationship between HRV parameters and CDAI appeared in the smooth curve, a recursive method was employed to determine the inflection point, optimizing the model’s likelihood (Liu et al., 2013). All statistical analyses were performed using R software packages (http://www.r-project.org, R Foundation) and EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Inc., Boston, MA). A P-value of less than 0.05 (two-sided) was considered indicative of statistical significance.
Results
Patients
Of the 92 patients with CD (68 males, 24 females) identified in the medical records, 10 were excluded: three (two males, one female) with incomplete medical histories, two males with arrhythmia, four (three males, one female) who had received glucocorticoids, antidepressants, and analgesics, and one male smoker. Consequently, 82 participants (60 males, 22 females) were enrolled in the study. (Fig. 1).
Figure 1: Study participants.
Patient characteristics
Patient characteristics are summarized in Table 2. The mean age of the patients was 28.84 ± 11.87 years old, and approximately 73.2% of them were male. The patients were divided into two groups based on CDAI: active CD, CDAI > 150 (mean 248.81 ± 83.27) and remission CD, CDAI ≤ 150 (mean 94.43 ± 33.24). No substantial variations were observed between the two groups in terms of age, gender, marital status, educational attainment, Montreal classification (including age at diagnosis, disease site, and disease behavior), disease duration, history of bowel resection, albumin levels, or white blood cell (WBC) count. However, a significantly greater number of patients in the remission group received biologics for treatment. Compared with remission group, the body mass index (BMI) and HRV parameters (including TP, LF, HF, SDNN) in active group were significantly lower, while heart rate (HR) and CRP level were significantly higher.
| Groups | CDAI > 150 (n = 51) | CDAI ≤ 150 (n = 31) | P-value |
|---|---|---|---|
| Age (years) | 28.96 ± 11.80 | 28.65 ± 12.18 | 0.908 |
| Gender (n, %) | 0.498 | ||
| Males | 36 (70.59%) | 24 (77.42%) | |
| Females | 15 (29.41%) | 7 (22.58%) | |
| BMI (kg/m2) | 17.97 ± 2.94 | 20.33 ± 3.55 | 0.002 |
| Marital status (n, %) | 0.986 | ||
| Unmarried | 33 (64.71%) | 20 (64.52%) | |
| Married | 18 (35.29%) | 11 (35.48%) | |
| Level of education (n, %) | 0.658 | ||
| Under middle school education | 12 (23.53%) | 8 (25.81%) | |
| Senior high school education | 11 (21.57%) | 9 (29.03%) | |
| University degree or equivalent | 28 (54.90%) | 14 (45.16%) | |
| Duration of disease (years) | 2.00 (1.00–6.00) | 2.00 (1.55–5.00) | 0.388 |
| History of bowel resection (n,%) | 0.877 | ||
| No | 37 (72.55%) | 22 (70.97%) | |
| Yes | 14 (27.45%) | 9 (29.03%) | |
| Medications (n, %) | <0.001 | ||
| Mesalamine | 37 (72.55%) | 8 (25.81%) | |
| Biologics | 12 (23.53%) | 20 (64.52%) | |
| Immunomodulators | 2 (3.92%) | 3 (9.68%) | |
| CDAI score | 248.81 ± 83.27 | 94.43 ± 33.24 | <0.001 |
| Age at diagnosis (n, %) | 1.000 | ||
| A1 | 9 (17.65%) | 5 (16.13%) | |
| A2 | 36 (70.59%) | 23 (74.19%) | |
| A3 | 6 (11.76%) | 3 (9.68%) | |
| Disease location (n, %) | 0.745 | ||
| L1 | 12 (23.53%) | 7 (22.58%) | |
| L2 | 4 (7.84%) | 3 (9.68%) | |
| L3 | 34 (66.67%) | 19 (61.29%) | |
| L4 | 1 (1.96%) | 2 (6.45%) | |
| Disease behavior (n,%) | 0.490 | ||
| B1 | 31 (60.78%) | 22 (70.97%) | |
| B2 | 8 (15.69%) | 5 (16.13%) | |
| B3 | 12 (23.53%) | 4 (12.90%) | |
| WBC (x109) | 8.02 ± 3.41 | 6.83 ± 1.85 | 0.079 |
| CRP (mg/L) | 17.00 (7.05–47.50) | 4.40 (0.65–11.10) | <0.001 |
| Albumin (g/L) | 34.95 ± 5.76 | 37.04 ± 6.38 | 0.131 |
| HR (beats per minute) | 89.93 ± 17.46 | 81.27 ± 13.88 | 0.022 |
| HRV indices | |||
| TP (ms2) | 871.72 (279.75–1595.97) | 1815.11 (804.70–3397.47) | 0.002 |
| LF (ms2) | 157.41 (61.66–444.74) | 378.64 (211.46–962.09) | 0.006 |
| HF (ms2) | 230.89 (36.09–450.41) | 460.36 (144.39–1048.96) | 0.008 |
| SDNN (ms) | 34.55 (18.54–44.11) | 48.22 (31.80–63.33) | 0.005 |
Note:
Abbreviations: CDAI, Crohn’s disease activity index; BMI, body mass index; WBC, white blood cell; CRP, C-reactive protein; HR, heart rate; HRV, heart rate variability; TP, total power; LF, low frequency power; HF, high frequency power; SDNN, standard deviation of R-R intervals.
Univariate analysis
The results of univariate analysis are shown in Table 3. Univariate analysis showed that BMI, HRV parameters, HR, CRP, WBC, and medications were related to the CDAI. However, gender, age, age at diagnosis, disease location, disease behavior, level of education, marital status, history of bowel resection, duration of disease, and albumin were not related to the CDAI.
| CDAI | Statistics | β (95% CI) | P-value |
|---|---|---|---|
| Age (years) | 28.84 ± 11.87 | 0.20 [−1.68 to 2.08] | 0.8325 |
| Gender (n, %) | |||
| Males | 60 (73.17%) | Ref | |
| Females | 22 (26.83%) | 18.60 [−31.27 to 68.47] | 0.4669 |
| BMI (kg/m2) | 18.87 ± 3.36 | −13.51 [−19.44 to −7.57] | <0.0001 |
| Marital status (n, %) | |||
| Unmarried | 53 (64.63%) | Ref | |
| Married | 29 (35.37%) | −14.90 [−61.16 to 31.35] | 0.5296 |
| Level of education (n, %) | |||
| Under middle school education | 20 (24.39%) | Ref | |
| Senior high school education | 20 (24.39%) | −19.50 [−82.98 to 43.97] | 0.5487 |
| University degree or equivalent | 42 (51.22%) | −28.17 [−82.70 to 26.37] | 0.3144 |
| Duration of disease (years) | 4.36 ± 5.11 | 0.88 [−3.48 to 5.24] | 0.6932 |
| History of bowel resection (n, %) | |||
| No | 59 (71.95%) | Ref | |
| Yes | 23 (28.05%) | 0.03 [−49.32 to 49.39] | 0.9989 |
| Medications (n, %) | |||
| Mesalamine | 45 (54.88%) | Ref | |
| Biologics | 32 (39.02%) | −97.41 [−138.87 to −55.94] | <0.0001 |
| Immunomodulators | 5 (6.10%) | −28.36 [−112.89 to 56.17] | 0.5128 |
| Age at diagnosis (n, %) | |||
| A1 | 14 (17.07%) | Ref | |
| A2 | 59 (71.95%) | −29.80 [−89.43 to 29.82] | 0.3302 |
| A3 | 9 (10.98%) | −8.44 [−94.13 to 77.26] | 0.8475 |
| Disease location (n, %) | |||
| L1 | 19 (23.17%) | Ref | |
| L2 | 7 (8.54%) | 28.30 [−58.89 to 115.50] | 0.5265 |
| L3 | 53 (64.63%) | 54.35 [1.62, 107.08] | 0.5265 |
| L4 | 3 (3.66%) | −12.19 [−134. to 70, 110.33] | 0.8459 |
| Disease behavior (n, %) | |||
| B1 | 53 (64.63%) | Ref | |
| B2 | 13 (15.85%) | −4.26 [−66.15 to 57.62] | 0.8929 |
| B3 | 16 (19.51%) | 35.58 [−21.45 to 92.62] | 0.2250 |
| WBC (x109) | 7.57 ± 2.96 | 9.32 [2.08 to 16.56] | 0.0136 |
| CRP (mg/L) | 24.94 ± 35.22 | 1.06 [0.47 to 1.65] | 0.0007 |
| Albumin (g/L) | 35.74 ± 6.05 | −2.68 [−6.32 to 0.96] | 0.1528 |
| HR (beats per minute) | 86.66 ± 16.66 | 2.50 [1.28 to 3.72] | 0.0001 |
| HRV indices | |||
| TP (ms2) | 1,746.84 ± 1,918.12 | −0.02 [−0.03 to −0.01] | 0.0025 |
| LF (ms2) | 535.83 ± 748.11 | −0.03 [−0.06 to 0.00] | 0.0025 |
| HF (ms2) | 538.76 ± 785.33 | −0.04 [−0.07 to −0.01] | 0.0047 |
| SDNN (ms) | 40.33 ± 21.82 | −1.86 [−2.80 to −0.92] | 0.0002 |
Note:
Abbreviations: CDAI, Crohn’s disease activity index; BMI, body mass index; WBC, white blood cell; CRP, C-reactive protein; HR, heart rate; HRV, heart rate variability; TP, total power; LF, low frequency power; HF, high frequency power; SDNN, standard deviation of R-R intervals.
Relations between HRV indices and CDAI
The relationship between HRV parameters and CDAI was assessed using a univariate linear regression model, with both unadjusted and adjusted model results presented in Table 4. In the unadjusted model, HRV parameters including TP, HF, and SDNN were negatively correlated with CDAI (β = −0.02, 95% confidence interval [CI] [−0.03 to −0.01], P = 0.0025; β = −0.04, 95% CI [−0.07 to −0.01], P = 0.0047; β = −1.86, 95% CI [−2.80 to −0.92], P = 0.0002, respectively). In the minimally adjusted model (adjusted for age and gender), the results did not change significantly (β = −0.02, 95% CI [−0.03 to −0.01], P = 0.0029; β = −0.04, 95% CI [−0.07 to −0.01], P = 0.0065; β = −2.02, 95% CI [−3.03 to −1.02], P = 0.0002, respectively). However, in the fully adjusted model (adjusted for age, gender, medications, BMI, history of bowel resection, and disease location), the negative correlations between TP, HF, and SDNN and CDAI were not significant (β = −0.01, 95% CI [−0.02 to 0.01], P = 0.3465; β = −0.01, 95% CI [−0.04 to 0.01], P = 0.3616; β = −0.94, 95% CI [−1.88 to 0.00], P = 0.0529).
| Variable | Unadjusted mode (β, 95% CI, P) | Minimally adjusted model (β, 95% CI, P) | Fully adjusted model (β, 95% CI, P) |
|---|---|---|---|
| TP | −0.02 [−0.03 to −0.01] 0.0025 | −0.02 [−0.03 to −0.01] 0.0029 | −0.01 [−0.02 to 0.01] 0.3465 |
| TP tertile | |||
| Low | Ref | Ref | Ref |
| Middle | −89.13 [−137.85 to −40.41] 0.0006 | −94.43 [−143.85 to −45.01] 0.0003 | −71.86 [−116.89 to −26.083] 0.0026 |
| High | −107.56 [−155.84 to −59.28] <0.0001 | −116.12 [−167.26 to −64.98] <0.0001 | −56.68 [−104.74 to −8.63] 0.0237 |
| P for trend | <0.0001 | <0.0001 | 0.0306 |
| LF | −0.03 [−0.06 to 0.00] 0.0645 | −0.03 [−0.06 to 0.00] 0.0837 | −0.00 [−0.03 to 0.02] 0.7983 |
| LF tertile | |||
| Low | Ref | Ref | Ref |
| Middle | −102.39 [−151.06 to −53.71] <0.0001 | −104.51 [−153.77 to −55.25] <0.0001 | −69.69 [−114.39 to −25.00] 0.0032 |
| High | −97.76 [−146.00 to −49.52] 0.0002 | −102.75 [−154.38 to −51.12] 0.0002 | −48.30 [−95.82 to −0.77] 0.0503 |
| P for trend | 0.0003 | 0.0002 | 0.0605 |
| HF | −0.04[−0.07 to −0.01] 0.0047 | −0.04 [−0.07 to −0.01] 0.0065 | −0.01 [−0.04 to 0.01] 0.3616 |
| HF tertile | |||
| Low | Ref | Ref | Ref |
| Middle | −67.65 [−118.52 to −16.78] 0.0109 | −72.58 [−124.97 to −20.19] 0.0082 | −44.53 [−89.02 to −0.05] 0.0537 |
| High | −90.42 [−140.84 to −40.01] 0.0007 | −95.63 [−149.17 to −42.10] 0.0008 | −47.68 [−94.91 to −0.45] 0.0518 |
| P for trend | 0.0008 | 0.0008 | 0.0543 |
| SDNN | −1.86 [−2.80 to −0.92] 0.0002 | −2.02 [−3.03 to −1.02] 0.0002 | −0.62 [−1.60 to 0.37] 0.2230 |
| SDNN tertile | |||
| Low | Ref | Ref | Ref |
| Middle | −67.68 [−117.36 to −18.00] 0.0092 | −77.18 [−128.59 to −25.77] 0.0043 | −69.39 [−114.24 to −24.55] 0.0034 |
| High | −104.55 [−153.79 to −55.31] <0.0001 | −115.74 [−168.54 to −62.94] <0.0001 | −64.73 [−111.61 to −17.84] 0.0086 |
| P for trend | <0.0001 | <0.0001 | 0.0101 |
Note:
Unadjusted model: we did not adjust other covariants Minimally adjusted model: we adjusted age and gender Fully adjusted model: we adjusted age, gender, medications, BMI, history of bowel resection,disease location CI, confidence interval; Ref, reference.
Abbreviations: CDAI, Crohn’s disease activity index; BMI, body mass index; HRV, heart rate variability; TP, total power; LF, low frequency power; HF, high frequency power; SDNN, standard deviation of R-R intervals.
In the unadjusted model, the negative correlation between HRV parameters, LF, and CDAI was not significant (β = −0.03, 95% CI [−0.06 to −0.00], P = 0.0645). There was no change in the minimally adjusted model (β = −0.03, 95% CI [−0.06 to −0.00], P = 0.0837). In the fully adjusted model, the negative correlation between LF and CDAI was still not significant (β = −0.00, 95% CI [−0.03 to 0.02], P = 0.7983).
For the trend test, TP, LF, HF, and SDNN were used as categorical variables (tertiles). The results showed that as the value of TP and SDNN increased the value of the CDAI decreased, and the negative correlation trends were significant (P = 0.0306 and P = 0.0101).
Analyses of non-linear relations
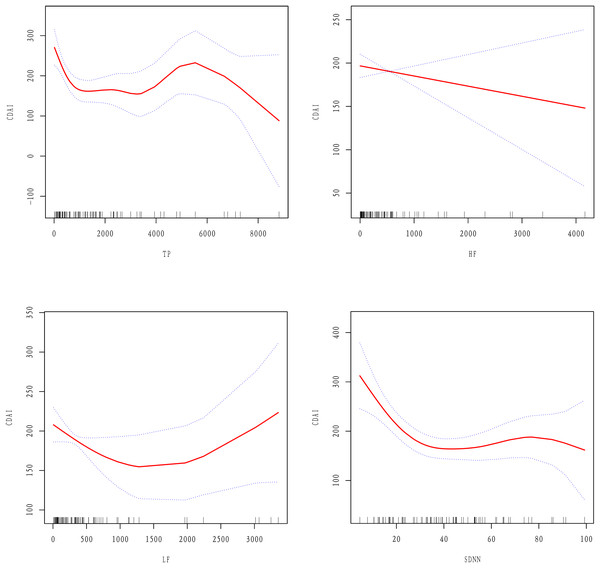
Since HRV parameters are continuous variables, it was necessary to perform non-linear relations analyses. The results showed that after adjusting for age, gender, BMI, disease location, history of bowel resection, and medications there were non-linear relations between TP, LF, SDNN, and the CDAI, while there was a linear relation between HF and the CDAI (Fig. 2).
Figure 2: Linear or non-linear relations between HRV parameters and the CDAI after adjusting for age, gender, BMI, medications, disease location, and history of bowel resection.
A two-piecewise linear regression model was employed to determine the inflection points of the curves (as shown in Table 5). For TP, the inflection point was observed at 246.68 ms2, with an effect value of −0.77 on the left side of the point (95% CI [−1.15 to −0.4], P = 0.0001). For LF, the inflection occurred at 60.74 ms2, where the effect value on the left side was −3.95(95% CI [−5.63 to −2.27], P = < 0.0001). For SDNN, the inflection point was at 28.63 ms, and the effect value to the left of this point was −6.74 (95% CI [−9.88 to −3.59], P < 0.0001). Notably, no correlation was found between TP, LF, SDNN, and the CDAI on the right side of the respective inflection points (β = −0.00, 95% CI [−0.01 to 0.01], P = 0.9523; β = 0.01, 95% CI [−0.02 to 0.03], P = 0.5170; β = 0.46, 95% CI [−0.67 to 1.59], P = 0.4237).
| Inflection point | Effect size (β) | 95% CI | P-value | |
|---|---|---|---|---|
| TP | ≤246.68 | −0.77 | [−1.15 to −0.40] | 0.0001 |
| >246.68 | −0.00 | [−0.01 to 0.01] | 0.9523 | |
| LF | ≤60.74 | −3.95 | [−5.63 to −2.27] | <0.0001 |
| >60.74 | 0.01 | [−0.02 to 0.03] | 0.5170 | |
| SDNN | ≤28.63 | −6.74 | [−9.88 to −3.59] | <0.0001 |
| >28.63 | 0.46 | [−0.67 to 1.59] | 0.4237 |
Note:
Abbreviations: CDAI, TP: total power; LF, low frequency power; SDNN, standard deviation of R-R intervals.
Discussion
The aim of this study was to explore the relations between HRV parameters and the CDAI. Through several adjustment models and trend tests, we observed that HRV parameters were generally negatively correlated with the CDAI, among which TP, HF, and SDNN were significantly negatively correlated with the CDAI. Smooth curve fitting showed that there was a linear relation between HF and the CDAI: as HF increased, the CDAI decreased. However, there were non-linear relations between TP, LF, and SDNN and the CDAI. On the left side of the inflection point, TP, LF, and SDNN increased while the CDAI decreased, but on the right side of the inflection point there were no significant correlations.
HRV is the most sensitive index that reflects autonomic nervous function. HRV parameters are mainly divided into the time domain indics and the frequency domain indics. The time domain index SDNN refers to the standard deviation of the RR interval, which reflects the total variability of the autonomic nervous system, including the sympathetic nerves and the vagus nerve (Berntson et al., 1997; Thayer, Yamamoto & Brosschot, 2010). In the frequency domain, high frequency power HF is recognized as a vagus nerve-mediated HRV parameter (Thayer, Yamamoto & Brosschot, 2010), while low frequency power LF is considered to represent sympathetic nerve activity. However, it is involved in the baroreflex mechanism regulated by sympathetic nerves and the vagal efferent nerve, so in theory it is also affected by the vagus nerve (Goldstein et al., 2011). TP refers to the total power, which is the sum of high frequency power (HF), low frequency power (LF), and very low frequency power (VLF) (Task force of the European society of cardiology and the North American society of pacing and electrophysiology, 1996). Each HRV parameter reflected the activity of different autonomic nerves, however, it might be due to vagus nerves (Thayer, Yamamoto & Brosschot, 2010). In this study, HRV parameters were in general negatively correlated with the CDAI, reflecting that autonomic nervous function decreased due to active inflammation. Among them, TP, HF, and SDNN, which are greatly influenced by the vagus nerve, showed significant and stable negative correlations with active inflammation. However, LF parameters, which mainly represent sympathetic nerve activity, showed no significant negative correlation with the CDAI, which confirmed the physiological relation between the vagus nerve and active inflammation (Tracey, 2002).
As the main HRV parameter mediated by vagus nerve, HF exhibited the most stable and strong negative correlation with CDAI in this study, which suggests that the vagus nerve might be the common mechanism of the continuous negative correlation between HRV and inflammation. SDNN and TP also exhibited a negative correlation with active inflammation, since they contain components of the vagus nerve. A meta-analysis in 2019 showed that HRV parameters were generally negatively correlated with inflammatory markers, among which HF and SDNN had the strongest correlation with inflammatory markers (Williams et al., 2019). The results suggested that HRV parameters could be used to index activity of the neurophysiological pathway responsible for adaptively regulating inflammatory processes in humans (Williams et al., 2019). Moreover, an earlier CD study showed that there was a negative relation between HF and serum tumor necrosis factor-alpha (TNF-α) levels. Compared with CD patients with low HF, CD patients with high HF had lower TNF-α levels6. Our results are basically consistent with these results, emphasizing the importance of the vagus nerve in regulating the inflammatory response of CD.
Previous studies have shown that the decline of autonomic nervous function in CD patients mainly presents as a sympathetic-vagal imbalance (Sharma et al., 2009; Kim, Yao & Ju, 2020; Aghdasi-Bornaun et al., 2018). Curve fitting showed that TP and SDNN had a relation with the CDAI, but their relation was not significant on the right side of the inflection point. Therefore, we speculate that the main reason for the sympathetic-vagal imbalance might be a decline of vagus nerve function rather than excessive sympathetic nervous function. This physiological imbalance provides a target for CD therapy; improving vagus nerve activity may help reduce inflammatory activity.
This study is the largest sample-sized investigation to date on the relationship between HRV parameters and the inflammatory activity of Crohn’s disease. However, there are some limitations to this study. Firstly, the research was not conducted in a blinded manner; instead, the HRV parameters were collected from the system’s backend by an operator independently. Secondly, since this study was cross-sectional, it does not allow for the establishment of causal relationships.
Conclusion
This study, utilizing generalized additive models and two-segment regression analysis, uncovers the numerical relationship between heart rate variability parameters and the CDAI, emphasizing the negative linear correlation between vagal nerve activity and inflammatory activity in CD. In summary, the findings of this study reveal an association between reduced vagal nerve activity and the disease activity in patients with CD. This breakthrough provides valuable quantitative metrics for monitoring inflammatory activity and lays the theoretical groundwork for the development of vagus nerve modulation therapies.