Microbial communities inhabiting the surface and gleba of white (Tuber magnatum) and black (Tuber macrosporum) truffles from Russia
- Published
- Accepted
- Received
- Academic Editor
- Rodolfo García-Contreras
- Subject Areas
- Biodiversity, Ecology, Microbiology, Mycology
- Keywords
- Truffles, Tuber macrosporum, Tuber magnatum, Metagenome, Geotrichum sp., Microbiota, Gleba, Surface of truffle
- Copyright
- © 2025 Malygina et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Microbial communities inhabiting the surface and gleba of white (Tuber magnatum) and black (Tuber macrosporum) truffles from Russia. PeerJ 13:e20037 https://doi.org/10.7717/peerj.20037
Abstract
The complex symbiotic relationships between truffles and their microbiota, coupled with their obligate mycorrhizal lifestyle, present significant challenges for obtaining axenic mycelium and achieving controlled cultivation. This study aimed to characterize the microbial communities within the surface and gleba of truffle ascomata using 16S and 18S rRNA gene sequencing and identify the taxonomic composition and ecological roles of these microbiota. Specimens of Tuber magnatum (white truffle) and Tuber macrosporum (smooth black truffle) were collected, with T. magnatum representing the first documented discovery of this species in Russia. Metabarcoding profiling identified both species-specific and shared microbial taxa, with the yeast-like fungus Geotrichum spp. emerging as a core symbiont in both truffle species. Its consistent detection in surface and gleba tissues suggests a critical role in mycorrhizal establishment and spore dispersal, potentially mediated by sulfur volatiles that attract mycophagous fauna. In T. magnatum, the bacterial community was dominated by Proteobacteria, particularly Alphaproteobacteria and Gammaproteobacteria, with the nitrogen-fixing genus Bradyrhizobium being especially abundant. The truffle microbiota predominantly comprised soil-derived microorganisms (e.g., nitrogen-fixing Rhizobiaceae spp., phenol-degrading Mycoplana spp.) and plant-associated symbionts (e.g., ectomycorrhizal Sebacina spp.), implicating these communities in nutrient cycling, xenobiotic degradation, and host plant interactions. By elucidating the taxonomic and functional profiles of truffle-associated microbiota, this study provides foundational insights into their ecological contributions. Chemical differences align with tissue-specific microbial communities, suggesting microenvironmental specialization in bioactive compound synthesis. These findings advance efforts to replicate critical symbiotic interactions in vitro, a prerequisite for developing sustainable cultivation protocols for T. magnatum and T. macrosporum under controlled conditions.
Introduction
Truffles are hypogeous fungi whose growth is contingent upon specific climatic and edaphic conditions (Robin, Goutal-Pousse & Le Tacon, 2016). These fungi predominantly thrive in temperate climates with distinct seasons, which provide the necessary temperature and moisture variations for their life cycle (Hall et al., 2017). Their distribution is intrinsically linked to the range of their obligate host plants (Gryndler et al., 2017; Zambonelli, Iotti & Hall, 2015). The cultivation of truffle fungi, particularly Tuber melanosporum Vittad. and Tuber aestivum Vittad., has a long-established history in Southern and Central Europe. Recent advancements in the understanding of truffle biology have led to improved control and standardization of cultivation techniques, enabling the successful cultivation of several truffle species outside their native ranges. This includes the gastronomically prized Tuber magnatum Pico.
Despite its high market value and successful cultivation in regions such as Africa, T. melanosporum remains the most widely cultivated truffle species globally. Its cultivation has expanded significantly beyond Europe, with successful introductions in Australia, Canada, Chile, China, New Zealand, South Africa, and the United States (Yan et al., 2017; Lemmond et al., 2023). In Russia, truffles are predominantly found in deciduous and mixed forests, with notable concentrations in Krasnodar, Transcaucasia, and the Tula and Oryol regions. Additional occurrences have been documented in forests near Moscow, St. Petersburg, Smolensk, and Belgorod, among other areas (Malygina et al., 2024a; Vishnevsky, 2018).
Truffles are highly valued not only for their unique flavor but also for their significant nutritional properties. The composition of their bioactive compounds varies depending on species and geographical origin. However, carbohydrates and proteins constitute the primary components, with truffles also being rich in essential minerals, dietary fiber, amino acids, fatty acids, and healthy fats (Panche, Diwan & Chandra, 2016).
Truffles are characterized by a high concentration of unsaturated fatty acids, particularly oleic and linoleic acids, which collectively account for over 60% of their total fatty acid profile (Splivallo et al., 2019; Ori, 2019; Morgunova et al., 2023). Linoleic acid, an essential fatty acid, serves as a precursor to 1-octen-3-ol, a key aromatic compound that contributes significantly to the distinctive aroma of truffles. Oleic acid, another prominent fatty acid in truffles, is associated with well-documented health benefits, including cholesterol reduction, cardiovascular protection, and potential anti-tumor activity (Splivallo et al., 2011). In addition to their fatty acid content, truffle fungi are known to synthesize a diverse array of bioactive compounds, such as ascorbic acid, ergosterol, phenols, flavonoids, terpenoids, and phytosterols. Among these, flavonoids are particularly noteworthy due to their antioxidant, anti-inflammatory, antimutagenic, and antitumor properties. Kaya & Akcura (2014) mentioned properties and biosynthetic capability rarely observed in other edible mushrooms. Analyses of three Chinese truffle species have further revealed a rich abundance of antioxidant compounds, including ascorbic acid, β-carotene, gallic acid, and rutin, which may provide protection against oxidative stress-related diseases (Lee et al., 2020). The biotechnological potential of truffles is substantial, with their associated microbiota representing a promising source for the discovery of novel therapeutic agents (Pavić et al., 2013). Secondary metabolites produced by these microorganisms hold significant promise for applications in medicine and biotechnology, highlighting their potential for future research and development (Leonardi et al., 2021).
Truffles harbor a diverse and complex microbial community, comprising bacteria, yeasts, and filamentous fungi, which colonize them throughout their life cycle. While certain microorganisms, such as Pseudomonas spp., yeasts, and some mold fungi, contribute to post-harvest spoilage, others, including Bacillus spp. and Listeria spp., are recognized as potential pathogens. Microbial colonization occurs in both the surface (outer shell) and gleba (inner part) of the truffle, with significant variations in microbiome composition observed between these two regions. Although the roles of these microorganisms in the truffle life cycle, nutrient acquisition, and flavor development are widely acknowledged, their specific functions and interactions remain largely undetermined (Yu et al., 2016; Wu, Meenu & Xu, 2021). It is hypothesized that bacteria, such as Pseudomona s spp. and members of the Enterobacteriaceae family, play a role in truffle development and maturation (Splivallo et al., 2015). Furthermore, the microbiome associated with truffles is implicated in the biosynthesis of their characteristic volatile organic compounds (Tejedor-Calvo et al., 2021; Vahdatzadeh & Splivallo, 2018; Vita et al., 2015). This hypothesis is supported by experimental studies demonstrating that yeasts isolated from T. melanosporum and T. magnatum can independently produce volatile organic compounds when cultured on a medium supplemented with L-methionine. Further research has revealed that thiophene volatiles in T. borchii (white truffle) are generated through the biotransformation of non-volatile precursors by the associated bacterial community, rather than by the truffle itself (Reyna & Garcia-Barreda, 2014). A significant contribution of the bacterial community to aroma formation has also been proposed for other truffle species, including T. melanosporum, T. magnatum, and T. aestivum (Hilszczańska et al., 2016; Buzzini et al., 2005).
Thus, the aim of this study was to identify truffle ascomata and investigate the truffle associated microbial composition of their distinct parts, specifically the surface and gleba, through metabarcoding sequencing of 16S and 18S rRNA genes. These findings are expected to provide valuable insights into the mechanisms underlying mycorrhiza formation and ascoma development in truffles, which may contribute to the establishment of optimal conditions for their controlled cultivation.
Materials & Methods
Sampling
Two species of true truffles belonging to the genus Tuber were selected for this study: the smooth black truffle (T. macrosporum) and the white truffle (T. magnatum). The ascomata of both species exhibited a globular morphology. The surface of T. magnatum was white and rough, while that of T. macrosporum was dark brown with a pyramidal, verrucose surface. Large ascomata (>4 cm in diameter) were selected for analysis. The gleba of T. magnatum was grey, whereas that of T. macrosporum was black, both displaying a porous structure with characteristic white, branching veins (Deveau et al., 2019).
Truffles were collected in a pine forest near Moldovanovka village (Krasnodar region, GPS coordinates: 44.461813, 38.856708) in August 2023. The ascomata were located using trained truffle-hunting dogs and carefully excavated with a rake to ensure their integrity. The study utilized whole and intact truffle specimens for analysis. Within 1–2 h after collection, the truffles were cleaned and rinsed with running tap water using a toothbrush. Two distinct parts of the truffle ascomata were selected for metabarcoding profiling: the surface layer (surface) and the central part (gleba). The ascomata were surface-sterilized with 70% ethyl alcohol and flamed using a Bunsen burner. The surface was then completely removed using a sterile grater, and approximately 0.5–0.7 mL of surface powder was collected for DNA isolation and sequencing. The truffles were subsequently broken open around the perimeter, and an equivalent volume of gleba was excised using a sterile scalpel. Tris-EDTA (TE) buffer (10 mM Tris–HCl, 1 mM EDTA) was added to the resulting samples prior to total DNA extraction.
Additionally, one of the minor objectives of the current study was to visualize the distinct composition of natural compounds in different parts of the ascocarps of T. macrosporum. For this purpose, natural compounds were extracted from distinct and freshly dissected parts of the ascoma using a general liquid extraction method with acetonitrile and methanol, followed by high performance liquid chromatography–mass spectrometry (HPLC-MS) analysis. Due to limitations in our ability to provide a detailed analysis, we are able to provide only general chromatographic profiles. A more detailed methodology can be found in the description of the figures included in the additional materials (Figs. S4–S5) (Pereliaeva et al., 2022; Morgunova et al., 2023).
DNA extraction
DNA was extracted from truffle ascomata using mechanical homogenization to disrupt the chitinous cell walls. Metal beads were added to the tubes containing the biomaterial suspended in TE buffer. The samples were homogenized using a BABR x1 vibrating grinder (Mycotech LLC, Irkutsk, Russia) at 3,000 rpm for one minute. This process was repeated three times, with cooling periods between each homogenization cycle (Malygina et al., 2024a; Malygina et al., 2024b).
Total DNA for metabarcoding profiling was extracted from crushed truffle ascomata using the DNeasy Plant Mini Kit (69106; QIAGEN) following the manufacturer’s recommendations (Tuovinen et al., 2019). The prepared samples, containing isolated DNA, were sent to BioSpark LLC (Troitsk, Russia) via express post at a temperature of 4 °C for metabarcoding profiling.
Amplification, sequencing, bioinformatic data processing
Metabarcoding profiling of truffle-associated microbial communities was performed through amplification of 16S rRNA gene fragments spanning the hypervariable V3–V4 regions using universal eubacterial primers. Fungal communities were analyzed by amplifying the internal transcribed spacer (ITS) regions, specifically targeting the ITS2 region and adjacent hypervariable regions of the 18S rRNA gene (Table 1). These primers are specific for the amplification of fungal DNA.
| Type of primer | Name | Sequence 5′–3′ | Concentration µM | Program of amplification |
|---|---|---|---|---|
| For 16S rRNA | ||||
| F | GPro341F | CCTACGGGNBGCASCAG | 0.625 | 95 °C–3 min (initial denaturation); 95 °C–30 s, 57 °C–30 s, 72 °C–30 s (35 cycles); 72 °C–5 min (final extension step). |
| R | GPro806R | GGACTACNVGGGTWTCTAATCC | 0.625 | |
| F | NR_16_341F1 | TCGTCGGCAGCGTCAGATGTGTATAAGAGACA GTGCCTACGGGNBGCASCAG | 2.5 | |
| F | NR_16_341F2 | TCGTCGGCAGCGTCAGATGTGTATAAGAGACA GCTGCCTACGGGNBGCASCAG | 2.5 | |
| F | NR_16_341F3 | TCGTCGGCAGCGTCAGATGTGTATAAGAGACA GTCTGCCTACGGGNBGCASCAG | 2.5 | |
| R | NR_16_806R1 | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGCC GGACTACNVGGGTWTCTAATCC | 2.5 | |
| R | NR_16_806R2 | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGA CCGGACTACNVGGGTWTCTAATCC | 2.5 | |
| R | NR_16_806R3 | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGAA CCGGACTACNVGGGTWTCTAATCC | 2.5 | |
| For 18S rRNA | ||||
| F | NR_5.8SR | TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGATCT CGATGAAGAACGCAGCG | 5.0 | 95 °C–3 min (initial denaturation); 95 °C–30 s, 55 °C–30 s, 72 °C–30 s (7 cycles); 72 °C–5 min (final extension step). |
| R | NR_ITS4R | GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGGCA TCCTCCGCTTATTGATATGC | 5.0 | |
Library preparation was conducted using the dual-index Nextera Index Kit (Illumina) following the manufacturer’s protocol (Hirose et al., 2020). Following PCR amplification, amplicons were purified using AMPure XP magnetic beads (KAPA Biosystems) to remove primer dimers and non-specific products. Purification steps were performed in accordance with the manufacturer’s guidelines and (Mahajan & McLellan, 2020).
Nucleotide sequences were subjected to shotgun metagenomic sequencing. High-throughput sequencing was performed on the Illumina MiSeq platform using paired-end sequencing with a minimum output of 10,000 paired-end reads per sample. Libraries were prepared and sequenced using the MiSeq Reagent Kit v2 Nano and MiSeq v2 Reagent Kit (500-cycle configuration) following the manufacturer’s protocols (Sato et al., 2019).
Bioinformatic data processing began with checking the quality of the raw readings. The quality assessment was performed using the FastQC program (v. 0.11.9 Andrews, 2010). Adapters from the sequence were cut using the following parameters: HEADCROP:20, CROP:221, LEADING:3, TRAILING:3/SLIDING WINDOW:5:10, AVGQUAL:20, MINLEN:221 (Chen et al., 2018). The length of the nucleotide sequences was 221 nucleotides. The data was then analyzed using the DADA2 algorithm (v. 1.26.0) in R (version 4.2.1). The reads were filtered, paired, chimeras were removed, and then a search was performed on the Silva database (for 16S - silva_nr99_v138.1_train_set, for 18S -SILVA_132_SSURef_tax_silva version).
Phylogenetic analysis
Phylogenetic analysis was conducted using MEGA X (Kumar et al., 2018). Nucleotide sequences were aligned with those displaying the highest similarity available in the NCBI database, utilizing the ClustalW algorithm with default parameters. Quality control check of the sequences was performed using UGENE (v. 39.0) (Okonechnikov et al., 2012). Sequences were aligned with the MUSCLE tool implemented in UGENE (v. 39.0) with default parameters. Pairwise distances were calculated using MEGA X with 100 bootstrap replications, uniform rates among sites, transitions and transversions were included, also we chose pairwise deletion for missing data (gaps).
The best model for phylogenetic analysis of Russian T. magnatum was K2 (Kimura2-parameter model) according to Bayesian information criterion (BIC) and was obtained by the Model Selection tool implemented in MEGA X with default parameters. Phylogenetic reconstruction was performed using the maximum likelihood method with 100 bootstrap replications in MEGA X. Visualization was performed with iTOL (Letunic & Bork, 2021). The best model for phylogenetic analysis of Russian T. macrosporum was T92+G according to BIC and was obtained by the Model Selection tool implemented in MEGA X with default parameters. Phylogenetic reconstruction was performed using the maximum likelihood method with 100 bootstrap replications in MEGA X. Visualization was performed with iTOL.
Statistics
Metabarcoding analysis was performed on three ascomata of T. macrosporum and four ascomata of T. magnatum. The reliability of the data was assessed using the Mann–Whitney U test. Statistical analysis was conducted using the software Past 4.10 (Natural History Museum, Oslo, Norway). Graphical representations were generated based on mean values ± standard deviations throughout the study.
Construction of co-occurrence networks of microbial communities
We developed a comprehensive Python pipeline for analyzing truffle-associated microbial communities, covering all stages from data processing to network visualization. The analysis began with construction of a ≪ sample × taxon≫ abundance matrix using pandas and numpy libraries. Microbial abundance data underwent centered log-ratio transformation for normalization, followed by removal of rare taxa (prevalence <10%) (Bars-Cortina, 2022).
Statistical analysis performed with scipy.stats included calculation of Pearson correlations for all taxon pairs, with p-value estimation and subsequent false discovery rate correction to minimize type I errors. Statistically significant correlations (|r| ≥ 0.1, p ≤ 0.05) were used to construct interaction networks via networks library (Ma et al., 2021), where taxa were represented as nodes and significant correlations as edges (red for positive, blue for negative associations).
Network topology was characterized using key metrics: modularity (for detecting functional communities), clustering coefficient, node centrality (degree centrality), along with within-module connectivity (Zi) and among-module connectivity (Pi) indices. Visualization implemented with matplotlib included: taxonomic coloring of nodes, size scaling by abundance or degree centrality, edge thickness proportional to correlation strength, force-directed layout optimization, and detailed legend creation using matplotlib.lines.Line2D.
Results
Identification and phylogenetic analysis
Several truffle species have been identified through molecular methods, including T. macrosporum and T. magnatum. Their genetic affiliation has been confirmed by phylogenetic analysis, which revealed well-defined clades consistent with other representatives of the same species. Notably, all three representative ascomata of T. macrosporum form a mixed clade on the phylogenetic tree, clustering with representatives of this species from different countries (Fig. S1). Additionally, this study reports the presence of T. magnatum Krasnodar region of Russia for the first time (Fig. S2), a species previously documented only in several European countries, such as Italy, France, and Croatia (Bach et al., 2021). The Russian T. magnatum specimens did not form independent clades and exhibited high genetic similarity with other representatives of T. magnatum (Fig. S3).
Truffle associated communities in the surface and gleba of T. macrosporum
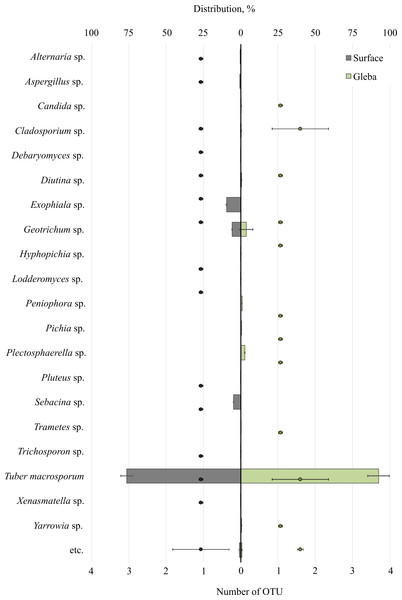
Metabarcoding profiling of the surface and gleba of the smooth black truffle T. macrosporum revealed a diverse eukaryotic community. Sequencing of the 18S rRNA gene identified a wide range of eukaryotic microorganisms within the ascoma. Significant differences in the composition of eukaryotic microbial communities were observed between the surface and gleba (Fig. 1).
Figure 1: The distribution of fungal communities inhabiting the ascomata of Tuber macrosporum is expressed in operational taxonomic units (OTUs, %).
The histogram represents the percentage ratio (upper scale), while the dots indicate the OTU values (lower scale). Error bars (whiskers) represent confidence intervals.The eukaryotic community inhabiting T. macrosporum (OTU 88.1 ± 3.9%) also included members of the phyla Ascomycota (OTU 12.02 ± 4.46%) and Basidiomycota (OTU 3.78 ± 2.11%), and was represented by 21 distinct fungal genera.
The eukaryotic microbial community within the surface of T. macrosporum ascomata included the following genera: Exophiala sp. (OTU 9.5%), Geotrichum sp. (OTU 5.77%), Sebacina sp. (OTU 4.9%), Aspergillus sp. (OTU 0.75%), Alternaria sp. (OTU 0.34%), Diutina sp. (OTU 0.3%), Xenasmatella sp. (OTU 0.18%), Cladosporium sp. (OTU 0.16%), Debaryomyces sp. (OTU 0.15%), Pluteus sp. (OTU 0.11%), Lodderomyces sp. (OTU 0.08%), Trichosporon sp. (OTU 0.06%), Hyphopichia sp. (OTU 0.05%).
The minor eukaryotic groups identified in the T. macrosporum ascoma sample included the following genera: Geotrichum sp. (OTU 3.6 ± 3.16%), Peniophora sp. (OTU 0.46 ± 0.46%), Cladosporium sp. (OTU 0.27 ± 0.16%), Diutina sp. (OTU 0.26 ± 0.3%), Yarrowia sp. (OTU 0.23 ± 0.22%), Candida sp. (OTU 0.19 ± 0.02%), Plectosphaerella sp. (OTU 0.14 ± 0.14%), Trametes sp. (OTU 0.07 ± 0.07%). Notably, the genus Geotrichum sp. was detected in both the surface sample and the truffle gleba of T. macrosporum.
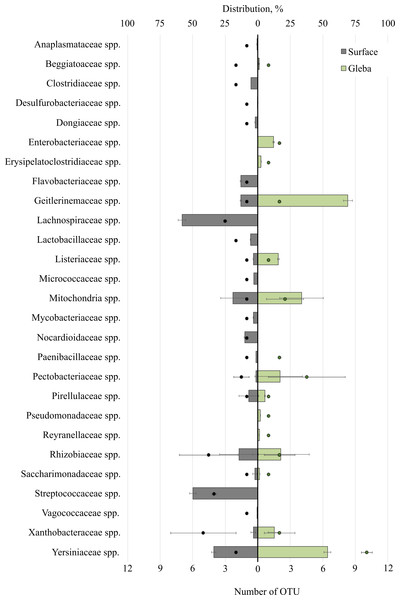
Analysis of bacterial communities inhabiting the surface and core of T. macrosporum ascomata revealed that the dominant classes were Alphaproteobacteria (OTU 31.01 ± 35.35%), Actinobacteria (OTU 8.22 ± 4.73%), Gammaproteobacteria (OTU 2 ± 24.92%), Bacilli (OTU 1.49 ± 20.21%), and Saccharimonadia (OTU 1.42 ± 1.23%). Minor classes, each constituting less than 1% of the community, included Clostridia, Cyanobacteriia, Planctomycetes, Bacteroidia, and Desulfurobacteriia.
The bacterial community within the surface of T. macrosporum ascomata comprised 27 families (Fig. 2). The dominant groups included the bacterial families Lachnospiraceae spp. (OTU 58.23%), Streptococcaceae spp. (OTU 49.75%), Yersiniaceae spp. (OTU 33.73), Mitochondria spp. (OTU 19.02 ± 32.78%), Rhizobiaceae spp. (OTU 14.38 ± 10.64%), Geitlerinemaceae spp. (OTU 12.99%), Flavobacteriaceae spp. (OTU 12.98), Nocardioidacea e spp. (OTU 10.11%). Minor groups, each constituting less than 10% of the community, included the following families: Lactobacillaceae spp. (OTU 5.63%), Clostridiaceae spp. (OTU 5.14%), Listeriaceae spp. (OTU 3.43%), Xanthobacteraceae spp. (OTU 3.39 ± 1.28%), Mycobacteriaceae spp. (OTU 3.37%), Micrococcaceae spp. (OTU 2.94%), Saccharimonadaceae spp. (OTU 2.26 ± 1.19%), Dongiaceae spp. (OTU 2.05%), Pectobacteriaceae spp. (OTU 1.48%), Pirellulaceae spp. (OTU 1.47%), Paenibacillaceae spp. (OTU 1.31%), Xanthobacteraceae spp. (OTU 1.28%), Beggiatoaceae spp. (OTU 1%), Anaplasmataceae spp. (OTU 0.71%), Vagococcaceae spp. (OTU 0.5%), Desulfurobacteriaceae spp. (OTU 0.1%).
Figure 2: The distribution of bacterial communities inhabiting the ascomata of Tuber macrosporum is expressed in operational taxonomic units (OTUs, %).
The histogram represents the percentage ratio (upper scale), while the dots indicate the OTU values (lower scale). Error bars (whiskers) represent confidence intervals.Thus, the unique families of bacteria for surface were Clostridiaceae spp., Dongiaceae spp., Flavobacteriaceae spp., Lachnospiraceae spp., Lactobacillaceae spp., Micrococcaceae spp., Mycobacteriaceae spp., Nocardioidaceae spp., Paenibacillaceae spp., Streptococcaceae spp., Vagococcaceae spp. Evaluation of the metagenome of the truffle fungus T. macrosporum revealed several genera of bacteria found only in its core Erysipelatoclostridiaceae spp. (OTU 12.21%), Pseudomonadaceae spp. (OTU 1.87%), Enterobacteriaceae spp. (OTU 1.48%), Reyranellaceae spp. (OTU 1.24%).
Truffle associated communities in the surface and gleba of T. magnatum
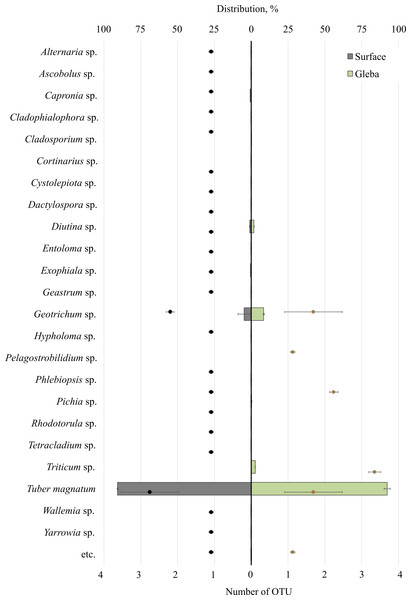
The community of eukaryotes inhabiting T. magnatum (OTU 90.71 ± 2.55%) additionally consisted of the phylum Ascomycota (OTU 8.9 ± 3.79%), Streptophyta (OTU 2.69%), Basidiomycota (OTU 0.29 ± 0.01%), Ciliophora (OTU 0.04%), and a proportion of undescribed taxa (OTU 0.08 ± 0.05%) (Fig. 3).
Figure 3: The distribution of eukaryotic microorganisms inhabiting the ascomata of Tuber magnatum is expressed in operational taxonomic units (OTUs, %).
The histogram represents the percentage ratio (upper scale), while the dots indicate the OTU values (lower scale). Error bars (whiskers) represent confidence intervals.Twenty-five genera represented the fungal community of T. magnatum. The surface sample of the ascoma of T. magnatum contained such genera as Geotrichum sp. (OTU 6.84 ± 0.67%), Diutina sp. (OTU 0.94 ± 0.23%), Capronia sp. (OTU 0.74%), Exophiala sp. (OTU 0.61%), Pichia sp. (OTU 0.17%), Rhodotorula sp. (OTU 0.16%), Cystolepiota sp. (OTU 0.13%), Geastrum sp. (OTU 0.11%), Phlebiopsis sp. (OTU 0.15%), Cladophialophora sp. (OTU 0.1%), Tetracladium sp. (OTU 0.1%), Serendipitacea e sp. (OTU 0.09%), Yarrowia sp. (OTU 0.09%), Ascobolus sp. (OTU 0.08%), Dactylospora sp. (OTU 0.06%), Alternaria sp. (OTU 0.05%), Hypholoma sp. (OTU 0.04%), Wallemia sp. (OTU 0.03%), Entoloma sp. (OTU 0.01%), Cortinarius sp. (OTU 0.01%).
Fungi of the following genera were found in the T. magnatum gleba‘s sample: Geotrichum sp. (OTU 5.44 ±3.25%), Triticum sp. (OTU 2.69%), Diutina sp. (OTU 0.93 ± 0.91%), Pichia sp. (OTU 0.13%), Cladosporium sp. (OTU 0.08%), Pelagostrobilidium sp. (OTU 0.04%).
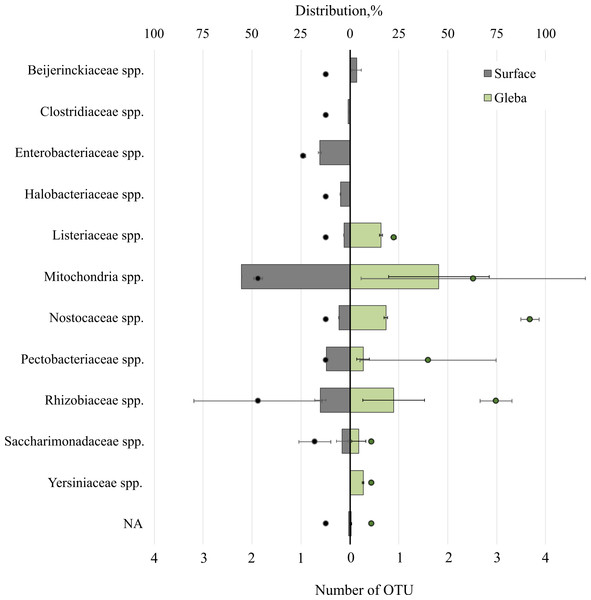
The T. magnatum truffle prokaryote community was represented by 11 families of bacteria (Fig. 4). The surface of the ascoma of T. magnatum included such families as Mitochondria spp. (OTU 55.39 ± 0.01%), Enterobacteriaceae spp. (OTU 15.37 ± 0.76%), Rhizobiaceae spp. (OTU 15.17 ± 2.07%), Pectobacteriaceae spp. (OTU 11.92%), Nostocaceae spp. (OTU 5.5 ± 0.1%), Halobacteriaceae spp. (OTU 4.77 ± 0.23%), Beijerinckiaceae sp. (OTU 3.4 ± 1.75%), Listeriaceae spp. (OTU 3.03%), Clostridiaceae spp. (OTU 0.83%), Saccharimonadaceae spp. (OTU 3.89 ± 2.15%).
Figure 4: The distribution of of bacterial communities inhabiting the ascomata of Tuber magnatum is expressed in operational taxonomic units (OTUs, %).
The histogram represents the percentage ratio (upper scale), while the dots indicate the OTU values (lower scale). Error bars (whiskers) represent confidence intervals.Analysis of the surface of the ascoma of T. magnatum has identified unique families of Clostridiaceae spp., Enterobacteriaceae spp., and assessment of the metagenome of the truffle fungus T. magnatum has revealed several genera of bacteria found only in its core Yersiniaceae spp. and Beijerinckiaceae spp.
Network analysis of truffle-associated microbial communities
This study presents a comparative network analysis of bacterial communities associated with smooth black (T. macrosporum) and white (T. magnatum) truffles, revealing distinct structural and functional patterns in their microbial and fungal consortia. The investigation employed correlation network construction and topological analysis using Zi-Pi metrics to characterize these complex microbial systems.
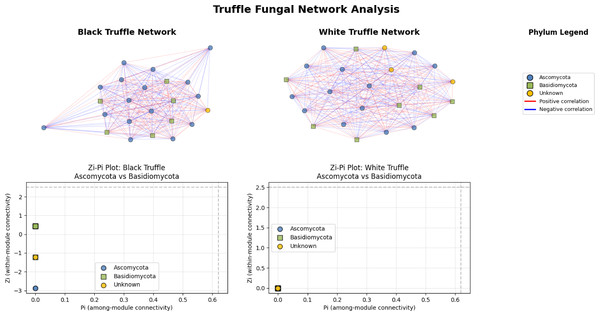
The fungal correlation networks of black and white truffles exhibit distinct structural patterns despite their similar composition (Fig. 5). The black truffle network consists of 23 nodes, each representing different fungal taxa, with node shapes and colors indicating phylogenetic affiliation: blue circles correspond to Ascomycota, green squares to Basidiomycota, and yellow circles to unknown taxa. Connections between nodes reflect statistically significant correlations, where red edges denote positive interactions and blue edges negative ones.
Figure 5: Comparative analysis of fungal topology in ascomata of smooth black (Tuber macrosporum) and white (Tuber magnatum) truffles.
In contrast, the white truffle network displays a comparable structure with 24 nodes but differs in connection patterns and taxonomic distribution. A key distinction lies in their topological properties: while the black truffle network shows 98.8% density (indicating extremely high connectivity) and a clustering coefficient of 98.9% (suggesting strong modularity), the white truffle network reaches full connectivity (100% density) with maximum clustering (100%), forming a uniformly interconnected structure.
Additionally, the black truffle network exhibits greater structural heterogeneity, as evidenced by the Zi (within-module connectivity) values ranging from −2.87 to 0.43, pointing to differentiated roles among taxa. In contrast, the white truffle network demonstrates complete structural homogeneity, with Zi = 0 for all nodes, implying a lack of specialized hub taxa. These differences suggest that black truffles harbor a more complex and modular fungal community, whereas white truffles maintain a uniformly interconnected microbiome with fewer structural subdivisions.
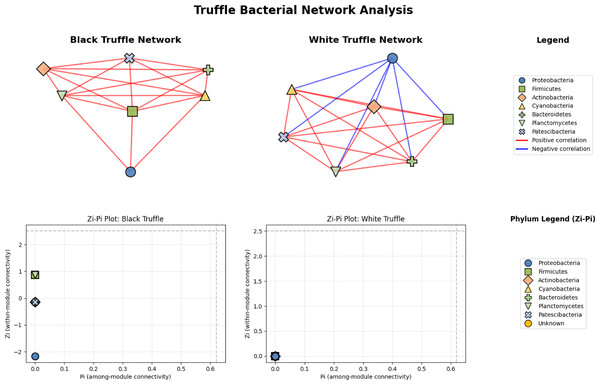
Network analysis reveals divergent structural architectures between black and white truffle-associated bacterial communities, notwithstanding their taxonomic similarities (Fig. 6). The black truffle dataset comprised samples containing seven bacterial phyla, forming a network with seven significant correlations. This network exhibited high modularity (0.4552), indicating clear separation into two distinct functional modules. Notably, the majority of inter-phylum connections were positive (red edges), suggesting predominantly cooperative relationships among bacterial groups. In contrast, the white truffle network, derived from samples of similar phylum diversity (seven phyla), showed greater complexity with 8 significant correlations and lower modularity (0.1710), organized into three communities with mixed interaction types—both positive (red) and negative (blue) correlations.
Figure 6: Comparative analysis of bacterial community topology in ascomata of smooth black (Tuber macrosporum) and white (Tuber magnatum) truffles.
Topological analysis through Zi-Pi plots revealed fundamental differences in network architecture. The black truffle network demonstrated a Zi range from −1.414 to 1.414, while all nodes showed Pi = 0, indicating complete absence of inter-module connectivity. This suggests a strictly compartmentalized structure where bacterial phyla operate within isolated functional units. Conversely, the white truffle network displayed greater topological diversity with Zi values ranging from −0.707 to 1.414 and Pi values up to 0.667, confirming the presence of connector nodes that bridge different modules.
Discussion
This study investigates the microbial community composition associated with white (T. magnatum) and smooth black (T. macrosporum) truffles. Using metabarcoding profiling of the surface and gleba tissues of ascomata, we aim to identify key microorganisms associated with their development and mycorrhizal formation. Despite their culinary and economic importance, controlled cultivation of truffles remains challenging due to their obligate symbiotic relationships with specific tree hosts. Characterizing the taxonomic and functional profiles of these microbial symbionts—as well as their ecological roles—is critical for advancing cultivation strategies for these high-value fungi.
This study builds upon prior investigations of truffle-associated microbial communities in Russia. We previously characterized the microbiota of T. aestivum (Malygina et al., 2024a) and identified biochemical and chemical peculiarities (Morgunova et al., 2023; Morgunova et al., 2024) as well as bioactive properties (Shelkovnikova et al., 2024; Vlasova et al., 2024). Here we report the first confirmed discovery of the prized white truffle T. magnatum in Russian territory (Fig. S2), representing a significant biogeographic expansion of this species’ known range. This finding is particularly noteworthy given T. magnatum’s traditionally restricted distribution across Southern and Central Europe, including its classic habitats in Italy, Croatia, Slovenia, and Hungary (Belfiori et al., 2020). While recent studies have documented its presence in more eastern locations such as Turkey (Doğan, Şen & Allı, 2024) and remarkably in tropical Thailand (Suwannarach et al., 2017), the occurrence of this ecologically specialized truffle in Russian ecosystems presents an unexpected extension of its biogeographic boundaries. Phylogenetic analysis revealed no genetic differences between Russian specimens and conspecific populations from other regions.
Truffles (genus Tuber) host diverse assemblages of truffle-associated microorganisms that fulfill critical ecological roles, including facilitating mycorrhizal symbiosis establishment and ascoma development. Previous studies have characterized microbial symbionts within truffle ascomata, host plant roots, and the mycorrhizosphere, with a focus on bacterial communities associated with Tuber aestivum, T. borchii, T. melanosporum, T. indicum, and T. magnatum (Antony-Babu et al., 2014; Barbieri et al., 2016; Perlińska-Lenart et al., 2020; Monaco et al., 2021; Sillo et al., 2022; Siebyła, Szyp-Borowska & Młodzińska, 2024). Fungal communities associated within truffles have also been reported, though to a lesser extent (Pacioni & Leonardi, 2016; Liu et al., 2020; Marozzi et al., 2023). However, systematic layer-by-layer microbial profiling of T. magnatum and T. macrosporum—spanning both surface and gleba tissues—has not yet been conducted, leaving a critical gap in understanding the spatial and functional organization of their microbiota.
Performed analysis revealed that truffle-associated microbial communities are mostly presented by aerobic microorganisms. Anaerobic taxa were predominantly detected in the surface of the T. macrosporum. Nitrogen-fixing bacteria and producers of organic sulfur compounds were ubiquitously distributed across all ascoma tissues. In contrast, known antibiotic-producing microorganisms and organic compound synthesizers were primarily localized to the surface. Metabarcoding profiling further indicated that the microbial consortia of these truffles comprise soil-derived taxa alongside phytopathogens. Notably, animal and human-associated pathogens were also identified, with the highest relative abundance observed in surface samples (Table 2).
| Kingdom | Taxon | Type of microorganism | Tuber magnatum | Tuber macrosporum | ||
|---|---|---|---|---|---|---|
| Surface | Gleba | Surface | Gleba | |||
| Bacteria | Beijerinckiaceae spp. | Gram-negative, aerobes, nitrogen-fixing, free-living, methanotrophs | + | + | ||
| Bacteria | Clostridiaceae spp. | Gram-positive, obligate anaerobes, are part of the normoflora of the GI tract, some human and animal pathogens | + | + | ||
| Bacteria | Enterobacteriaceae spp. | Gram-negative, aerobes. Pathogens and producers of extended-spectrum β-lactamases, carbapenemases and L-histidine | + | + | ||
| Bacteria | Erysipelatoclostridiaceae spp. | Gram-positive, aerobes or facultative anaerobes, animal and human pathogens, some representatives inhabit the human gut microflora | + | |||
| Bacteria | Flavobacteriaceae spp. | Gram-negative, aerobes, some representatives of facultative anaerobes, fish pathogens | + | |||
| Bacteria | Geitlerinemaceae spp. | Cyanobacteria, photosynthesising. | + | + | ||
| Bacteria | Halobacteriaceae spp. | Archaea, most Gram-positive, mostly aerobes, extremal halophiles, free-living saprophytes | + | |||
| Bacteria | Listeriaceae spp. | Gram-positive, aerobes, microaerophiles. Human and animal pathogens | + | + | + | + |
| Bacteria | Lachnospiraceae spp. | Gram-positive, obligate-anaerobic, inhabit the intestinal microflora of humans and animals, saprophytes (process lignocellulose and carbon dioxide), produce butyric acid | + | |||
| Bacteria | Lactobacillaceae spp. | Gram-positive, facultatively anaerobic or microaerophilic, probiotics in human and animal gut microflora, produce lactic acid, participate in food fermentation | + | |||
| Bacteria | Micrococcaceae spp. | Gram-positive, aerobes or facultative anaerobes, there are a small number of species classified as obligate anaerobes, saprophytes, pathogens | + | |||
| Bacteria | Mycobacteriaceae spp. | Gram-positive, aerobes, acid and alcohol tolerance, saprophytes, human and animal pathogens | + | |||
| Bacteria | Nocardioidaceae spp. | Gram-positive, aerobes, saprotrophs, bioindicators of gas hydrate deposits | + | |||
| Bacteria | Paenibacillaceae spp. | Gram-positive, aerobes or facultative anaerobes, plant symbionts, nitrogen fixation, antibiotic producers, used as pesticides | + | |||
| Bacteria | Pectobacteriaceae spp. | Gram-negative, facultative anaerobes, pectolytic. Plant pathogens | + | + | + | + |
| Bacteria | Pirellulaceae spp. | Gram-negative, aerobes, microaerophiles or anaerobes | + | + | ||
| Bacteria | Pseudomonadaceae spp. | Gram-negative, aerobes, human and plant pathogens, saprotrophs, some species synthesise antibiotics and biopesticides | + | |||
| Bacteria | Rhizobiaceae spp. | Gram-negative, aerobic, nitrogen-fixing. Symbiotic bacteria (symbiosis with leguminous plants). Some species are plant pathogens | + | + | + | + |
| Bacteria | Saccharimonadaceae spp. | Obligate epibionts (symbionts of other bacteria), possible role in the gut microbiome | + | + | + | + |
| Bacteria | Streptococcaceae spp. | Gram-positive, facultative anaerobes, animal and human pathogens, probiotics (used in lactase deficiency), used in the dairy industry | + | |||
| Bacteria | Xanthobacteraceae spp. | Gram-negative, aerobes, plant symbionts, nitrogen fixers | + | |||
| Bacteria | Yersiniaceae spp. | Gram-negative, facultative anaerobes. Human and animal pathogens | + | + | ||
| Fungi | Aspergillus sp. | Aerobes, saprotrophs, producers of enzymes, antibiotics, production of organic acids (citric acid, gluconic acid), human and animal pathogens | + | |||
| Fungi | Capronia sp. | Aerobes, micromycetes, saprotrophs, symbionts (some form associations with lichens), human and animal pathogens, black yeasts | + | |||
| Fungi | Diutina sp. | Facultative anaerobes, yeasts, human pathogens | + | |||
| Fungi | Exophiala sp. | Aerobes, micro-mycetes, polyextremophilic opportunistic pathogen, black yeast | + | + | ||
| Fungi | Geotrichum sp. | Aerobes, saprotrophs, micromycetes, found in normal human microflora, producer of volatile organic sulphur compounds | + | + | + | + |
| Fungi | Sebacina sp. | Aerobes, saprotrophs, symbionts (mycorrhizae) | + | |||
| Fungi | Plectosphaerella sp. | Aerobes, saprotrophs, phytopathogens, micromycetes | + | |||
Notes:
“+” means presence of microorganism.
Metabarcoding analysis of eukaryotic communities in smooth black (T. macrosporu m) and white truffles (T. magnatum) revealed the consistent presence of Geotrichum spp. as a dominant fungal genus in both the surface and gleba of ascomata. Members of Geotrichum are implicated in the production of volatile sulfur compounds, which contribute to the characteristic aroma of truffles (Caboni et al., 2020). These compounds, such as dimethyl trisulfide, are derived from the enzymatic catabolism of L-methionine (Splivallo, 2008). For instance, the soil fungus Geotrichum candidum Link, 1,809 metabolizes methionine into volatile derivatives through enzymatic activity (Bonnarme et al., 2001; Vahdatzadeh & Splivallo, 2018). Such compounds act as olfactory attractants for mycophagous animals (e.g., wild boars), which consume truffles and disperse their spores via fecal deposition. This symbiotic interaction facilitates spore dissemination and subsequent mycorrhizal colonization of host plant roots. The surface-specific enrichment of Geotrichum spp. suggests their potential role in mediating interactions between truffles and soil fauna. Critically, the absence of Geotrichum spp. in the soil microbiome may impair ascoma formation, as these fungi likely support truffle development through both biochemical signaling and ecological facilitation of spore dispersal.
Metabarcoding analysis revealed distinct eukaryotic communities in T. macrosporum, with Exophiala spp. and Sebacina spp. dominating the surface, while Plectosphaerella spp. and Peniophora spp. were characteristic of the gleba. Exophiala spp., known endophytes associated with Quercus ilex L. roots colonized by T. melanosporum (Herrero de Aza et al., 2022), and Sebacina spp., ectomycorrhizal fungi forming tripartite symbioses with host plants and other root-associated fungi (including Pezizales truffles) suggest roles in mediating symbiotic interactions (Murat et al., 2008; Leonardi et al., 2013; Marjanović et al., 2020). In contrast, Plectosphaerella spp. and Peniophora spp., phytopathogens linked to root rot in crops (Carlucci et al., 2012; Lambevska, Rusevska & Karadelev, 2013), likely represent transient colonizers. Notably, Peniophora cinerea (Pers.), detected in T. borchii ascomata, was tested for mycorrhizal involvement via co-cultivation with Populus alb a seedlings and T. borchii mycelium. Histological analysis using deoxynucleotidyl transferase labeling revealed no apoptosis, tannin deposition, or detectable hyphal colonization in roots, indicating no functional role in mycorrhization (Ragnelli et al., 2014; Pacioni & Leonardi, 2016). Also, metabarcoding profiling identified Alternaria spp., Ascobolus spp., Wallemia spp., and Yarrowia spp. as dominant eukaryotic taxa in the surface of T. magnatum. Like Exophiala spp., Alternaria spp. are endophytes previously detected on Quercus spp. roots colonized by T. melanosporum (Herrero de Aza et al., 2022). Ascobolus spp., coprophilous fungi dependent on herbivore-mediated spore dispersal, derive nutrients from undigested plant matter in ruminant manure (Miyunga, 2015). Wallemia spp., first identified in soils surrounding the Chinese truffle Tuber indicum (Li et al., 2018), and Yarrowi a spp., soil-dwelling yeasts known to synthesize volatile organic sulfur compounds, are linked to the distinct aroma profile of T. magnatum (Splivallo, 2008).
Despite the use of primers specific for fungal DNA in this study, we were able to obtain sequences from representatives of the Triticum genus (family Poaceae) from the gleba of T. magnatum. The genus Triticum spp. combines species related to cereal plants. This observation likely reflects extracellular DNA incorporation during truffle development rather than a true symbiotic association. As discomycetes, truffles develop ascomata entirely underground, where they interact with and encapsulate organic and inorganic soil components. During maturation, soil-derived genetic material—including plant DNA from nearby flora—may passively integrate into the gleba matrix. In this case, Triticum spp. DNA likely originated from cereal plants growing in the forest ecosystem adjacent to the truffle’s habitat. While such incidental DNA uptake is a documented artifact in soil metagenomes, it underscores the importance of rigorous contamination controls when interpreting environmental sequencing data.
Metabarcoding profiling revealed that T. magnatum (white truffle) and T. macrosporum (smooth black truffle) share dominant prokaryotic taxa, including Rhizobiaceae (Alphaproteobacteria; Phyllobacterium, Rhizobium, Mycoplana), Yersiniaceae (Gammaproteobacteria), and Rickettsiales (Alphaproteobacteria), ubiquitously distributed across surface and gleba tissues. While Rickettsiales sequences were detected, their unresolved genus-level taxonomy complicates ecological interpretation. Rhizobium cf. leguminosarum uniquely induced hyphal vacuolization in T. aestivu m co-cultures, whereas Phyllobacterium showed no interaction, and Mycoplana—known for phenol biodegradation—may assist in degrading soil phenanthrenes to support ascoma development (Gryndler & Hrselova, 2012; Barbieri et al., 2016). The nitrogen-fixing capacity of Rhizobiaceae suggests a role in host plant symbiosis, while Geitlerinemataceae (cyanobacteria), dominant in T. macrosporum, likely enhance soil nitrogen cycling as biofertilizers (Rubin-Blum et al., 2024). Furthermore, metabarcoding of the T. macrosporum gleba revealed the presence of the family Pseudomonadaceae (Gammaproteobacteria), specifically bacteria of the genus Pseudomonas spp. Members of the Pseudomonas genus are frequently detected within truffle ascomata and play significant ecological roles. Microbes associated with the truffle ascoma are involved in its developmental processes (Chen et al., 2019). Research by Ballestra et al. (2010) has also implicated this bacterial genus in the spoilage of black truffles, even during extended low-temperature storage.
In this study, we also present some initial data, visualizing the mass spectrometric profiles characterizing the natural product content of the surface and gleba of the truffle T. macrosporum (Fig. S4–S5). In addition to the distinct microbial composition of different parts of the ascocarps, we detected differences in the natural product composition in distant parts of the ascomata. These differences were observed under varying extraction protocols and solvents.
Thus, metabarcoding profiling of the Russian truffles T. macrosporum and T. magnatum identified dominant bacterial and fungal taxa, including soil-derived microorganisms, plant symbionts, and phytopathogens. These microbial communities appear to facilitate key stages of truffle development, such as nutrient acquisition and mycorrhizal colonization. For instance, nitrogen-fixing bacteria (Rhizobiaceae spp.), phenol-degrading taxa (Mycoplana spp.), and cyanobacterial biofertilizers (Geitlerinemataceae spp.) likely enhance nutrient cycling in the rhizosphere, while fungal symbionts (Sebacina spp., Exophiala spp.) mediate interactions with host plant roots. Notably, the majority of identified microorganisms may contribute to enzymatic degradation of root cell wall components, enabling truffles to establish symbiotic interfaces for nutrient exchange. This functional synergy underscores the ecological interdependence between truffles and their microbiota, which collectively support fungal proliferation in soil ecosystems.
Our study reveals striking differences in microbial network architectures between white (T. magnatum) and smooth black truffles (T. macrosporum), providing new insights into their distinct ecological strategies. The T. magnatum microbiome forms a completely connected network (100% density), indicating exceptionally stable and comprehensive fungal associations that likely contribute to its renowned ecological specificity. In contrast, T. macrosporum maintains a slightly less dense network (98.8% density) but with greater structural complexity, evidenced by variable within-module connectivity (Zi range: −2.87 to 0.43) that suggests more specialized microbial partnerships (Antony-Babu et al., 2014; Monaco et al., 2022).
Both species maintain single, non-modular communities typical of closely related fungal consortia in truffle ascomata, yet they achieve this through different topological configurations. While T. magnatum shows remarkable homogeneity in node roles (all Zi = 0), T. macrosporum exhibits niche differentiation among microbial partners, potentially reflecting adaptation to more variable environmental conditions. These architectural differences likely stem from species-specific evolutionary pressures, including distinct habitat requirements, divergent host plant interactions, unique metabolic constraints, and varying ecological niches (Splivallo et al., 2015).
A particularly significant finding is the exclusive presence of bacterial connector taxa in T. magnatum, which appear to facilitate cross-species communication and metabolic integration—a feature conspicuously absents in T. macrosporum. This suggests T. magnatum has evolved greater dependence on microbial mediation for nutrient acquisition and cycling, possibly explaining its more restricted geographic distribution and habitat specificity compared to T. macrosporum.
The ecological and practical implications of these findings are substantial. For truffle cultivation, our results indicate that T. magnatum requires microbiome management strategies focused on maintaining network stability, while T. macrosporum may benefit from approaches that preserve its specialized microbial interactions. These findings demonstrating that phylogenetic proximity does not necessarily predict microbial community structure. The distinct network topologies we observed highlight the importance of considering species-specific microbial association patterns in truffle ecophysiology research and underscore the value of network analysis for developing targeted cultivation techniques that respect each species’ unique microbial ecology.
Conclusions
Consequently, this study reports the first documented discovery and characterization of T. magnatum in Russia. Metabarcoding profiling of T. magnatum and T. macrosporum revealed both species-specific and shared microbial taxa, enabling predictions about their functional and chemical roles in truffle biology. Notably, Geotrichum spp. emerged as a putative symbiotic partner common to both species, detected in both surface and gleba tissues. This ubiquitous distribution suggests a critical role in mycorrhizal symbiosis establishment and spore dispersal, potentially mediated by volatile sulfur compounds that attract mycophagous animals. In T. magnatum, the bacterial community was dominated by Proteobacteria, particularly Alphaproteobacteria and Gammaproteobacteria, with the nitrogen-fixing genus Bradyrhizobium being especially abundant.
The truffle-associated microbiome predominantly comprises soil-derived microorganisms and plant symbionts, including nitrogen-fixing bacteria (Rhizobiaceae spp.), phenol-degrading taxa (Mycoplana spp.), and cyanobacterial biofertilizers (Geitlerinemataceae spp.). These communities likely facilitate nutrient acquisition, organic compound degradation, and soil fertility enhancement—processes essential for ascoma development. By delineating the taxonomic and functional profiles of these microbiota, this work advances understanding of truffle ecology and provides actionable insights for optimizing cultivation strategies under controlled conditions.
Supplemental Information
The phylogenetic tree of T. macrosporum is presented, with orange colour indicating the sequences of strains identified in Russia
The phylogenetic tree of T. magnatum is presented, with special markers in the form of black dots indicating the sequences of strains identified in Russia
Typical MS-profile of natural products extracted by acetonitrile from different distant part of truffle Tuber macrosporum
For the extraction, 0.5 g of each truffle tissue sample was ground into a powder using a mortar and pestle with the addition of acetonitrile in a 1:10 ratio (w/v; weight of tissue to volume of solvent). The resulting homogenate was centrifuged for 10 min at 3,000× g rpm (Armed LC04B, St. Petersburg, Russia). Then, 800 µL of the supernatant was transferred into microtubes, and protein precipitation was performed by adding 80 µL of a 10% trichloroacetic acid solution. The microtubes were centrifuged again for 10 min at 16,000× g rpm (Biosan Microspin-12, Riga, Latvia). The prepared samples were stored at +4 °C. Prior to analysis, the samples were filtered through a 13 mm syringe filter with 45 µm pores and a polyvinylidene fluoride (PVDF) membrane, after which 100 µL of the filtrate was transferred into chromatographic vials. The extracts were then diluted with 900 µL of acetonitrile. Chromatographic determination was performed using ultra-high-performance liquid chromatography coupled with a triple quadrupole mass spectrometer (6470, Agilent Technologies, Germany) and an analytical Zorbax SB-Aq column (4.6 × 50 mm, 5 µm). The chromatographic conditions were set using deionized water/formic acid (99.9:0.1, v/v) (solvent A) and methanol/formic acid (99.9:0.1, v/v) (solvent B). The chromatographic separation of target compounds was carried out according to the following gradient (time (minutes), %B): (0, 30%); (3, 10%); (4, 30%). The injection volume and flow rate were 3 µL and 0.8 mL/min, respectively. Data acquisition and processing were performed using MassHunter software version B.08.00 (Agilent Technologies, Germany).
Typical MS-profile of natural products extracted by methanol from different distant part of truffle Tuber macrosporum
For extraction, 0.5 g of each truffle tissue sample was ground into a powder using a mortar and pestle with the addition of methanol (“Vecton”, Russia) in a 1:10 ratio (w/v; weight of tissue to volume of solvent). The resulting mixture was incubated for one hour on a roller mixer (MX-T6-S, BIOBASE, Jinan, China) and then centrifuged (LC-04A, Armed, Russia) at 3,000 rpm for 10 min. The supernatant was transferred into vials for chromatographic analysis. Chromatographic determination was performed using ultra-high-performance liquid chromatography (UHPLC) coupled with a triple quadrupole mass spectrometer (6470, Agilent Technologies, Germany) and an analytical Zorbax SB-Aq column (4.6 × 50 mm, 5 µm). The chromatographic conditions employed deionized water/formic acid (99.9:0.1, v/v) as solvent A and methanol/formic acid (99.9:0.1, v/v) as solvent B. Chromatographic separation of the target compounds was achieved using the following gradient program (time (min), %B): (0, 30%); (3, 10%); (4, 30%). The injection volume and flow rate were 3 µL and 0.8 mL/min, respectively. Data acquisition and processing were performed using MassHunter software (version B.08.00, Agilent Technologies, Germany).
Initial data from NGS sequencing of Tuber magnatum and Tuber macrosporum truffles
The initial data demonstrates the successful sequencing of all symbiont species of truffle fungi, as well as the differences in the composition of microorganisms between the peridium and the gleba. Additionally, mass spectrometric analysis confirms the differences in chemical composition between these two parts of the truffle.