A revision of Haematodes Laporte and Weiserianum Bernhauer (Coleoptera: Staphylinidae: Staphylininae: Xanthopygina)
- Published
- Accepted
- Received
- Academic Editor
- Marcio Pie
- Subject Areas
- Entomology, Taxonomy
- Keywords
- Rove beetles, Neotropical region, Myrmecophily, Morphology, New species
- Licence
- This is an open access article distributed under the terms of the Open Government License.
- Cite this article
- 2018. A revision of Haematodes Laporte and Weiserianum Bernhauer (Coleoptera: Staphylinidae: Staphylininae: Xanthopygina) PeerJ 6:e4582 https://doi.org/10.7717/peerj.4582
Abstract
The species of poorly known but charismatic genera Haematodes Laporte, 1835 and Weiserianum Bernhauer, 1927 are revised. Weiserianum syn. nov. is considered a junior synonym of Haematodes, with Haematodes kuntzeni (Scheerpeltz, 1936) comb. nov. Weiserianum woltersi Bernhauer, 1927 syn. nov. is treated as a synonym of Haematodes tenuipes Kraatz, 1858. Haematodes myteros sp. nov., is described from Paraguay and Brazil. As the type series of Haematodes bicolor Laporte, 1835 is considered lost, a neotype, selected from the original type locality is designated. We also designate a lectotype for H. tenuipes Kraatz, 1858 to stabilize nomenclature for this species, which is similar to H. myteros. As far as known, Haematodes is restricted to the southern Neotropical region and may be nest parasites within Acromyrmex and Atta ant nests as are species of the related genus Scariphaeus, but no direct observations are yet available. We provide a key to the four known species of Haematodes and illustrate their diagnostic features.
Introduction
The genera Haematodes Laporte and Weiserianum Bernhauer (Staphylininae: Staphylinini: Xanthopygina) contain four obscure and similar species with remarkable morphology, especially their distinctive antennae that are reminiscent of the ant nest beetles (Carabidae: Paussinae: Paussini) (Figs. 1, 2A–2C). These beetles were so notable that Nordmann (1837) even created the family group name Platycnemini (emended by Newton (1995) from the original Platycnemidiformes) for his new genus Platycnemus (maintained here as a synonym of Haematodes). Likely based on the broadly shaped pronotum and wide neck, Haematodes was included in Kraatz’s (1857) ‘Quediiformes’ along with other, distantly related Staphylininae, now known to belong to other subtribes (Brunke et al., 2016). Haematodes and Weiserianum were maintained in a loosely defined Quediina (e.g., Herman (2001)) until Chatzimanolis (2014a) unambiguously treated them as members of the Neotropical rove beetle subtribe Xanthopygina. Within this lineage, they have been hypothesized to be most closely related to the myrmecophile Scariphaeus Erichson (Scheerpeltz, 1936) or the suspected myrmecophile/sphecophile Darwinilus Chatzimanolis (Chatzimanolis, 2014b).
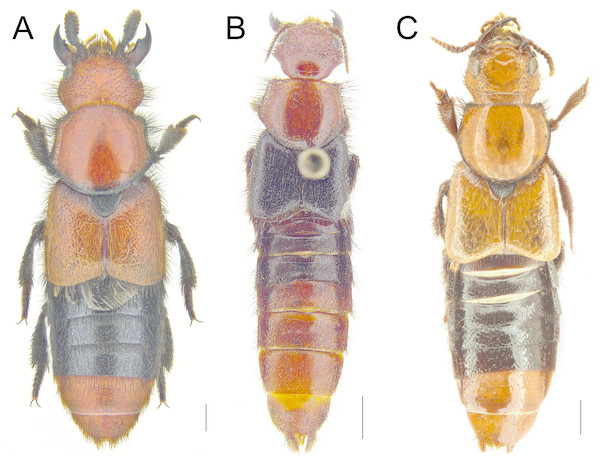
Figure 1: Habitus of Haematodes species.
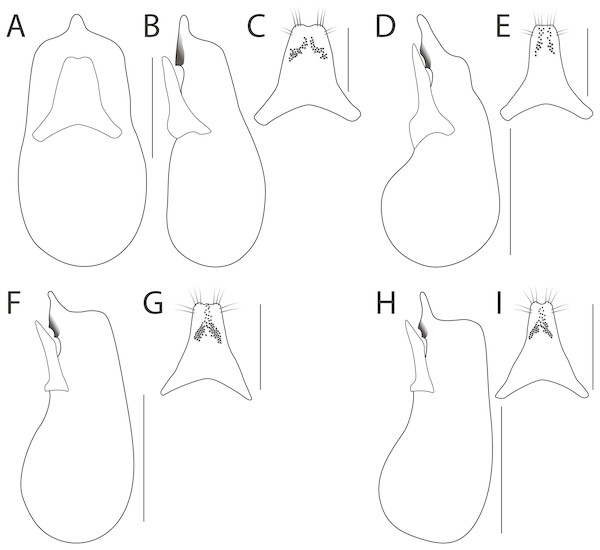
Haematodes bicolor Laporte (A), H. kuntzeni (Scheerpeltz) (B), H. tenuipes (Kraatz) (C). Scale bar = 2 mm. Images by the authors.Figure 2: Antennae, dorsal head, metatarsus and male sternite VII in Haematodes species.
Haematodes bicolor Laporte (A, D, E, G), H. myteros Brunke and Chatzimanolis (B, I), H. kuntzeni (Scheerpeltz) (C, H) and H. tenuipes Kraatz (F). Scale bars: 1 mm (A–C, E–F), 2 mm (D, G–I). Images by the authors.Specimens of these genera are rarely collected and are scattered throughout collections worldwide. The species are limited to the southern edge of tropical South America, the ‘Chacoan subregion’ of Morrone (2014), an area with little recent collection activity and that has been devastated by agricultural development. Morphological features such as thickened legs and antennae, elongate maxillae, and clusters of bristle-like hairs on the pronotum are found in other Staphylinini (e.g., Smilax, Chatzimanolis, 2016), and beetles in general (Parker, 2016), known to be associated with colonies of ants. Scheerpeltz (1937) recognized the close morphological similarity of Haematodes bicolor Laporte to Weiserianum woltersi Bernhauer and his new species, W. kuntzeni, stating that the former differed mainly by the more broadly expanded and flattened hind tarsi, the shorter apical maxillary palpomere and the more compact antennae. These charismatic taxa have never been comprehensively revised and nothing substantial has been published since Scheerpeltz (1937). We here illustrate the diagnostic features of and provide a key to four valid species of this lineage, one of which was new to science.
Materials and Methods
Specimens. Specimens were examined using Nikon SMZ 25 and Olympus SZX10 stereomicroscopes. Genitalia were washed with 70% alcohol and placed in glycerin for observation. Genitalia were placed in glycerin filled vials for long-term storage, which were pinned with their respective specimen. We followed the morphological terminology used in recent revisions of Xanthopygina (e.g., Chatzimanolis, 2016).
Measurements were performed using the live measurement module in NIS Elements BR v4.5. Measurements were taken as listed below, but only proportional (HW/ HL, PW/PL, PW/HW) and forebody measurements were stated directly in descriptions due to variability in body size. Total body length is generally difficult to measure accurately in Staphylinidae due to the contractile nature of the abdomen. Abbreviations for measurements are as follows:
- HL
-
Head Length, at middle, from the anterior margin of frons to the nuchal ridge.
- HW
-
Head Width, the greatest width, including the eyes.
- PL
-
Pronotum Length, at middle.
- PW
-
Pronotum Width, greatest width.
- EL
-
Elytral Length, greatest length taken from level of the anterior most large lateral macroseta to apex of elytra. Its length approximates the length of the elytra not covered by the pronotum and therefore contributing to the forebody length.
- Forebody
-
HL + PL + EL.
Photomontage was accomplished using a motorized Nikon SMZ25 microscope and NIS Elements BR v4.5. Photos were post processed in Adobe Photoshop CC. Line illustrations were performed in Adobe Illustrator CC based on photographs. Plates were assembled in Adobe InDesign CC and exported first to PDF, then to .tiff format. Maps were made using SimpleMappr (Shorthouse, 2010), with vague, country-level or state/department-level records omitted.
Specimens were examined from the following collections:
- AMNH
-
American Museum of Natural History, New York, NY, USA (L. Herman).
- CMNH
-
Carnegie Museum of Natural History, Pittsburg, PA, USA (R. Davidson).
- CNC
-
Canadian National Collection of Insects, Arachnids and Nematodes, Ottawa, ON, Canada.
- FMNH
-
Field Museum of Natural History, IL, USA. (C. Maier, M. Thayer, A. Newton).
- MZH
-
Zoological Museum, Finnish Museum of Natural History, University of Helsinki, Helsinki, Finland (M. Jaako).
- NMW
-
Naturhistorisches Museum, Vienna, Austria (H. Schillhammer).
- NMNH
-
National Museum of Natural History, Smithsonian Institution, Washington D.C., USA (F. Shockley, D. Furth).
- SDEI
-
Senckenberg Deutsches Entomologisches Institut, Müncheberg, Germany (L. Behne).
- SEMC
-
Snow Entomological Collection, Biodiversity Institute, Lawrence, KS, USA (Z. Falin).
- TAMU
-
Texas A&M University, TX, USA (Karen Wright).
- ZMHB
-
Museum für Naturkunde der Humboldt-Universität, Berlin, Germany (B. Jaeger).
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: E494A584-3784-45A8-8D0A-6C0E024EA5C8. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
Taxonomy
| Haematodes Laporte, 1835 |
| Haematodes Laporte, 1835: 112 [type species: H. bicolor Laporte, fixed by monotypy]. |
| Platycnemus Nordmann, 1837: 135 [type species: P. lateritius Nordmann, fixed by monotypy]. |
| Weiserianum Bernhauer, 1927: 247 syn. nov., [type species: W. woltersi Bernhauer, fixed by monotypy]. |
Type species. Haematodes bicolor Laporte.
Diagnosis. Among genera of Xanthopygina, Haematodes is easily recognized based on the distinct habitus that includes reddish to orange-red coloration and a ‘fuzzy’ appearance, due to the presence of long, bristle-like setae. It can be distinguished from the (presumably closely related) genera Scariphaeus Erichson and Smilax Laporte based on the combination of the following: antennae forming a long and broad but loose club of transverse segments 3–11 or 5–11 (Figs. 2A–2C); broad neck; abdominal tergites with only anterior transverse carinae, lacking posterior curved lines.
Redescription. Habitus as in Figs. 1A–1C; medium large and robust rove beetles; forebody 6.20–10.00 mm long. Body mostly reddish to light orange-red, with darker areas; elytra reddish to entirely dark, abdomen bicolored with basal 2–4 visible segments darker than apical ones. In species with more than a few specimens known, reddish color was observed to vary from dark red to pale orange-red.
Dorsal forebody without microsculpture, except for lateral punctate areas of the pronotum. Head transverse, somewhat hexagonal, with variably dilated temples, disc entirely punctate or with small glabrous area in center of disc, temples with several to many long bristle-like setae. Eyes ranging from 1/3 to 1/2 the length of the head. Ventral head with shallow to strong microsculpture; infraorbital ridge well developed, extending from base of mandible to connect with nuchal ridge (if present) and/or dorsal basal ridge, post-mandibular ridge absent; gular sutures separated but only narrowly at middle; nuchal ridge present in its entirety or in lateral fragments (in specimens of H. tenuipes, H. myteros) or entirely absent (H. bicolor, H. kuntzeni); neck broad in all species. Antennae short (Figs. 2A–2C), antennomere 1 compact, with many long bristle-like setae, at least twice as wide as antennomere 2; segments 3–10 or 5–10 extremely transverse, slightly asymmetrical and glabrous at middle, forming a long club with apical antennomere; antennomeres 5–11 or 6–11 with tomentose pubescence. Labrum well developed, moderately to deeply incised, margins with extensive (H. bicolor) to moderate amounts of long bristle-like setae. Mandibles moderately long and slender, roughly symmetrical, with a single tooth, with dorso-lateral mandibular groove. Maxilla with galea long, longer than maxillary palpus (Fig. 2D), maxillary palpus 4-segmented; P2-P 3 subequal, P4 slightly narrower and longer than P3, P2 with bristle-like setae. Labium entire, with small apical notch; labial palpi 3-segmented, P3 distinctly narrower and longer than P2, P2 with bristle-like setae; P3 apically truncate.
Pronotum (Figs. 3A–3D) wider than head; margin explanate; without post-coxal process; hypomeron not visible in lateral view; inferior line not connected anteriorly to superior line and joining anterior margin of pronotum (inferior line interrupted by coxal cavity in H. bicolor); margin of pronotum, especially anterior angles, with long bristle-like setae; basisternum with multiple macrosetae; prosternum without longitudinal carina. Elytra longer than pronotum, with dense to sparse, uniform punctation. Hind wings fully developed, with MP4 and CuA separate and MP3 present. Scutellum with posterior ridge, densely punctate and setose. Basal elytral ridge present, sinuate, directed anteriad. Humeral row of setae absent. Mesocoxal cavities broadly separated by a depressed intercoxal region, mesocoxal and metacoxal processes short and obtuse to nearly truncate. Legs with 5 − 5 − 5 tarsal segmentation; femora, tibia and tarsi flattened; tibia greatly expanded and with several rows of thick spines along outer face; tibia with two long apical spurs, unequal in length; protarsi enlarged and with tenent setae in both sexes; tarsomeres with middle of disc glabrous; empodium with pair of setae.
Figure 3: Pronotum of Haematodes species.
Haematodes bicolor Laporte (A), H. kuntzeni (Scheerpeltz) (B), H. tenuipes Kraatz (C), and H. myteros Brunke and Chatzimanolis (D). Scale bar = 2 mm. Images by the authors.Abdomen with paired prototergal glands present; abdominal terga with only anterior basal lines (curved and accessory basal lines absent). Abdominal sternite III with transverse basal line forming obtuse angle at middle, not sharply extended posteriad; male abdominal sternite VII with expanded porose structure, situated at midlength of sternite, bearing a dense brush of setae (Figs. 2G–2I); apex of male abdominal sternite VIII with broad, shallow emargination; male abdominal sternite IX robust, with moderately deep apical emargination. Aedeagus short and compact relative to most Xanthopygina; median lobe with one or two teeth more strongly sclerotized than surrounding tissue; paramere short and stout, always distinctly shorter than median lobe, with peg setae. Female with spermatheca not sclerotized.
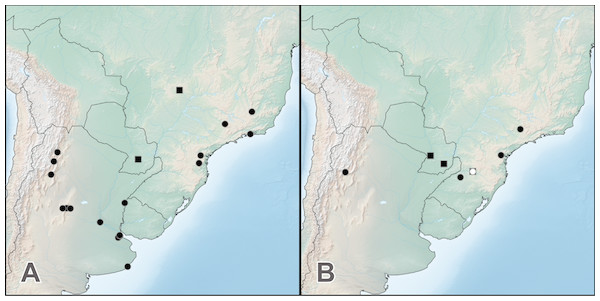
Distribution. Distributed in the Chacoan subregion of South America (Morrone, 2014): northern and central Argentina, northeast to Uruguay, Paraguay and southern Brazil. Distributions of Haematodes species, including those that are each others’ closest relatives, at least partially overlap. This phenomenon suggests processes other than allopatric speciation acting in this genus, perhaps those related to their potential host ants. This distribution pattern also occurs in the suspected sphecophile/myrmecophile Trigonopselaphus (Chatzimanolis, 2015), which has a similar distribution in the Chacoan subregion.
Biology. Little is known about species of Haematodes. One specimen of H. bicolor was collected in flight in a ‘scrub forest’. One specimen of H. tenuipes was collected at an Agricultural Research Station and may be associated with ants that nest underground in open-habitats, such as the grass-harvesting species of Atta. Nearly all specimens are loaded with soil particles, further supporting a ground-nesting host. Finally, the putatively related genus Scariphaeus is associated with Acromyrmex ants (Scheerpeltz, 1936), has a similar distribution and also often bears soil particles. Scheerpeltz (1937) suggested that the beetles may be associated with Hymenoptera, especially wasps but the morphology of the legs and antennae suggest that ants are far more likely.
Comments. The species of Haematodes and Weiserianum form a morphological grade with regard to features that are likely related to interactions with social insects (thicker appendages, long bristle-like setae, reductions of the nuchal ridge). The type species of Weiserianum, W. woltersi Bernhauer, was discovered to be a synonym of H. tenuipes Kraatz (see below). Although it would be possible to restrict Haematodes to its distinctive type species and move H. tenuipes to Weiserianum, we prefer to treat these taxa as a single, well-defined genus, easily separated from the related, myrmecophile genus Scariphaeus by the distinctive antennae and habitus. This runs in contrast to the usual tendency of taxonomists studying myrmecophiles to erect or maintain genera representing the steps along this grade (e.g., clavigerine Pselaphinae with successively fewer antennomeres). Therefore Weiserianum syn. nov. is considered to be a synonym of Haematodes. We maintain the synonymy of Platycnemus with Haematodes since their type species each correspond to the same taxonomic concept given here for H. bicolor.
Key to species of Haematodes Laporte
1. Antennae extremely broadened into a strong club (Fig. 2A); pronotum broadly glabrous dorsally (Fig. 3A); hind tarsi markedly robust (Fig. 2E); median lobe with one tooth in lateral view (Fig. 4B)…H. bicolor Laporte
Figure 4: Aedeagus in dorsal (A) and lateral (B, D, F, H) view, and paramere (C, E, G, I) of Haematodes species.
Haematodes bicolor Laporte (A–C); H. kuntzeni (Scheerpeltz) (D–E); H. tenuipes Kraatz (F–G); and H. myteros Brunke and Chatzimanolis (H–I). Scale bars = 1 mm (A–B, D, F, H), 0.5 mm (C, E, G, I). Illustrations by the authors.- Antennomeres broadened but forming only a weak club (Figs. 2B–2C); pronotum with at least a few dorsal punctures, often in loose rows (Figs. 3B–3D); hind tarsi slightly robust (Fig. 2F); median lobe with two teeth in lateral view (Figs. 4D, 4F, 4H)…2
2. Elytra dark, apical four abdominal segments reddish (Fig. 1B); nuchal ridge entirely absent; pronotum with a pair of broad, loosely organized punctate areas (Fig. 3B); paramere with apex truncate, peg setae in two rows, that do not fuse at middle (Fig. 4E)…H. kuntzeni (Scheerpeltz)
- Elytra and apical two abdominal segments reddish (Fig. 1C); nuchal ridge either complete or with lateral fragments, never absent; pronotum with a pair of punctate areas consisting of single, doubled or tripled rows, or disc of pronotum with only a few punctures (Figs. 3C–3D); paramere with apex emarginate or weakly trilobate, peg setae in two groups that fuse at middle (Figs. 4G, 4I)…3
3. Pronotum appearing subquadrate, strongly convex (Fig. 3D); apex of paramere with emargination simple (Fig. 4I); median lobe in lateral view with apex elongate (Fig. 4H)…H. myteros Brunke and Chatzimanolis
- Pronotum appearing transverse, middle portion weakly flattened (Fig. 3C); apex of paramere weakly trilobed (Fig. 4G); median lobe in lateral view with apex short and constricted (Fig. 4F)…H. tenuipes Kraatz
| Haematodes bicolor Laporte, 1835 |
| (Figs. 1A; 2A, 2D, 2E, 2G; 3A; 4A– 4C; 5A (map)) |
| Haematodes bicolor Laporte, 1835: 113. |
| Platycnemus lateritius Nordmann, 1837: 135 |
Type locality. Buenos Aires, Argentina.
Type material. Neotype (male, dissected, CNC), here designated: ARGENTINA; Prov. Bs. Aires, Olivos, XII.1976, Bolle [white printed label] / NEOTYPE Haematodes bicolor Laporte des. Brunke and Chatzimanolis 2018 [red printed label].
Laporte did not indicate the number of specimens studied, nor a type series but indicated that specimens came from Buenos Aires. The type material described by Laporte (1835) is considered lost (K Walker, Museum Victoria, pers. comm., 2018) as these specimens were among those destroyed by a fire at the Smithsonian Institution in 1865 (see Evenhuis, 2012). The second Laporte collection in Museum Victoria, Australia does not contain the type but only a drawing of the type by Laporte (K Walker, Museum Victoria, pers. comm., 2018), which, although crude, matches our species concept for H. bicolor regarding the greatly expanded tibiae. Additional attempts to locate the type series in the Natural History Museum London (M Barclay, pers. comm., 2018) and Oxford University (D Mann, pers. comm., 2018) have failed. Therefore, we found it necessary to designate a neotype to fix the identity of this taxon. A male was selected from the type locality (Buenos Aires, Argentina) and deposited at the CNC.
It is obvious from the descriptions of the genus and species (antennae, body form) by Laporte (1835) that he was referring to the species concept here given for H. bicolor.
| Platycnemus lateritius Nordmann, 1837 |
Type locality. ‘Brasilia meridionali’ (=southern Brazil).
Type material. Syntypes (2 #f, ZMHB): 5871 [typed label] / south Brasil, Sello, S. [handwritten label] / lateritius Typus Nordm. [handwritten red label] / bicolor Laporte, Pl. lateritius [handwritten green label] / Orig. of Tr. 47, 340 [handwritten label] / Haematodes Laporte, Platycnemus Nord. [handwritten label] / Hist. Coll. (Coleoptera), No. 5871, Haematodes bicolor Lap., Pl. lateritius N., x Brasil, Sello, Zool. Mus. Berlin [printed green label] / Haematodes bicolor Cast. [handwritten label] / SYNTYPE Platycnemus lateritius Nordman, 1837, labelled by MFNB 2017 [printed red label].
S. Brasil, Sello, S. [handwritten label] / Orig. of Tr. 47, 340 [handwritten label] / Hist. Coll. (Coleoptera), No. 5871, Haematodes bicolor Lap., Pl. lateritius N., x Brasil, Sello, Zool. Mus. Berlin [printed green label] / SYNTYPE Platycnemus lateritius Nordman, 1837, labelled by MFNB 2017 [printed red label].
Nordmann (1837) did not specify the number of specimens that he studied but referenced specimens collected by D. Sello from southern Brazil. Two syntypes matching this description (except S. rather than D. Sello) were located in ZMHB but we refrain from designating a lectotype as both are females and the identity of this taxon is not problematic.
Other material. ARGENTINA: Province unknown: coll. Schubert (1, ZMHB); Buenos Aires: Buenos Aires, 15.XII.1950 (1, CNC); Buenos Aires, IX.1897, G. Schimpf (1, ZMHB); Mar del Plata (1, CNC); Olivos, XII.1976, Bolle (21, CNC); Victoria, 3.II.1937 (1, CNC); Catamarca: Chaquiago, canal bank, 21.XII.1973, J.L. Neff (2, TAMU); unknown location, Björgesten, GAC.7929 (1, MZH); unknown location, 1939, M.J. Viana, SM0046428 (1, SEMC); Cordoba: Cordoba, San Vicente, J. Fenzel S. (1, ZMHB); Sierra de Cordoba, J. Fenzel S. (1, ZMHB); Cordoba, J. Fenzel S. (1, ZMHB); Tanti, II.1977, Bolle (11, CNC); Entre Rios: Chajais, XI.1975, Bolle (3, CNC); Salta: unknown location, 1905, Steinbach (2, ZMHB); Rosaria de Lerma, 1,325 m, 27–30.I.1982, H. & A. Howden (1, CNC) Yacochuya, 8 km NW Cafayate, 2.i.1972, D.J. Brothers (1, AMNH); Sante Fe: Rosario, Hübricht S [?] (1, ZMHB). BRAZIL: Mato Grosso do Sul: unknown location, Rhode, S. (2, ZMHB); Minas Gerais: Ouro Prêto, 12–13.IV.1968, F.C. Val (1, CNC); Poços de Caldas, O. Leoncini, 1968 (1, CNC); Paraná: Curitiba, 16.xii.1960, N. Marston (1, AMNH); Rio de Janeiro: unknown location, Gyllhl., Kymell, GAC.7943 (1, MZH); unknown location (2, CMNH); unknown location, coll. Schubert (1, ZMHB); Santa Cruz, Dr. Hensel (1, ZMHB); Santa Catarina: Rio Negrinho, G. Schauer (1, ZMHB). PARAGUAY: Cordillera: Instituto Agronomico Nacional Caacupe, 10.II.1981, R.D. Cave (1, NMNH). URUGUAY: unknown locality, R.O. Del, SM0046429 (1, SEMC). VENEZUELA: coll. Schubert (2, ZHMB) [likely mislabeled!].
Diagnosis. This species is easily recognized by the glabrous disc of the pronotum (Fig. 3A) and the strongly clubbed antennae (Fig. 2A).
Redescription. Forebody length 8.39–10.00 mm. Body reddish orange to yellow orange, appendages, lateral margins of pronotum, scutellum and abdominal segments III–VI dark brown to black.
Head transverse; temples greatly dilated, nuchal ridge absent, width/length ratio = 1.54–1.75, widest in posterior two-thirds, dorsal surface moderately densely punctate, with variably sized glabrous area at middle (Fig. 2D); eyes small, about one-third of lateral head length. Antennae extremely short and compact, all segments expanded and inflated, segments 4–10 strongly transverse, 5–9 slightly asymmetrical, tomentose pubescence missing medially on segments 5–10, segments 1–5 with bristle-like setae latero-apically (Fig. 2A), apical segment distinctly transverse and with weakly emarginate apex. Legs with tibia markedly expanded and triangular; hind tarsi broadened and greatly flattened (Fig. 2E). Pronotum width/head width ratio = 1.14–1.28, weakly transverse, width/length ratio = 1.23–1.37; disc of pronotum broadly glabrous, areas near margins, especially anterior angles, extensively punctate (Fig. 3A); punctation of elytra and abdomen fine and dense (Fig. 1A).
Male sternite VII with porose structure as in Fig. 2G, broad and thicker than in other species. Male sternite VIII with shallow emargination. Aedeagus as in Figs. 4A–4C. In ventral view, median lobe weakly converging apicad to blunt median projection. In lateral view, median lobe with single tooth and nearly flat ventral face (Fig. 4B). Paramere with apex emarginate and with two groups of peg setae, not distinctly fused together apically (Fig. 4C).
Distribution. Widely distributed across the entire range of the genus (Fig. 5A), H. bicolor is the most frequently collected species. Known from southern Brazil, Paraguay, Uruguay and northern and central Argentina, from lowlands to foothills of the Andes (1,325 m) (Fig. 5A). The two historical specimens labeled ‘Venesuela’ in ZMHB are obviously mislabeled.
Figure 5: Distribution of Haematodes species.
Haematodes bicolor Laporte (circles) and H. kuntzeni (Scheerpeltz) (squares) (A); and H. tenuipes (Kraatz) (circles) and H. myteros Brunke and Chatzimanolis (squares) (B). Map created by SimpleMappr (simplemappr.net).Bionomics. Laporte (1835) mentioned that his specimen (s) was taken on the wing from the Buenos Aires area in scrub habitat (= “on la prend volant sur les broussailles”).
| Haematodes kuntzeni (Scheerpeltz, 1936) comb. nov. |
| (Figs. 1B; 2C, 2H; 3B; 4D– 4E; 5A (map)) |
| Weiserianum kuntzeni Scheerpeltz, 1936 : 348 |
Type locality. Upper Rio Claro, ‘Jatahy’ (=Jataí), Goiás, Brazil.
Type material. Holotype (male, ZMHB): [white card missing mounted genitalia] / [male symbol] [white card] / Brasilien, Goyaz, Jatahy [green printed label] / TYPUS Weiserianum kuntzeni O. Scheerpeltz [red printed label] / Aedoeagus missing, labelled by MFNB January 2018 [white printed label] / Weiserianum kuntzeni, Typus, nov. spec. [handwritten label] / Weiserianum kuntzeni Scheerpeltz HOLOTYPE det. Brunke and Chatzimanolis 2018 [printed red label] / Haematodes kuntzeni (Kraatz) det. Brunke and Chatzimanolis 2018 [printed white label].
Other material. BRAZIL: unknown state: Chapada campo (1, CMNH). PARAGUAY: Guaira: Villa Rica, III.1939, Fr. Schade [marked incorrectly as typus] (1, NMW).
Diagnosis. This species is easily recognized by the dark elytra alone (Fig. 1B).
Redescription. Forebody length 6.98–7.97 mm. Body dark reddish orange, antenna brown, elytra, scutellum, legs, lateral margin of pronotum and abdominal segments III–IV black.
Head transverse, temples moderately expanded behind eyes, nuchal ridge absent, width/length ratio = 1.35–1.51, widest just anteriad of neck (marked by glabrous area), dorsal surface moderately densely punctate, without glabrous area at middle; eyes nearly half of head length. Antennae short, segments 4–10 strongly transverse and forming an elongate club (Fig. 2C), segments 5–10 slightly asymmetrical, tomentose pubescence missing medially on segments 5–10, segments 1–3 with bristle-like setae latero-apically (Fig. 2C), apical segment elongate and with weakly emarginate apex. Legs with tibia expanded and elongate triangular; hind tarsi slightly broadened and slightly flattened (as in Fig. 2F). Pronotum width/head width ratio = 1.29–1.35, strongly transverse (Fig. 3B), width/length ratio = 1.24–1.29; disc of pronotum with punctures loosely arranged into dorsal and sublateral longitudinal groups (Fig. 3B), anterior angles and lateral margins with extensive long and bristle-like setae (Fig. 3B); punctation of elytra and abdomen fine and dense (Fig. 1B).
Male sternite VII with porose structure as in Fig. 2H, wider than in other species. Male sternite VIII with shallow emargination. Aedeagus as in Figs. 4D–4E. In lateral view, median lobe with two distantly separated subapical teeth and long, relatively broad apical portion (Fig. 4D). Paramere with apex truncate and with two groups of peg setae, distinctly separated (Fig. 4E).
Distribution. Known only from Goiás state in Brazil and Guaira department in Paraguay (Fig. 5A).
Bionomics. Nothing is known about this species’ biology.
| Haematodes tenuipes Kraatz, 1858 |
| (Figs. 1C; 2F; 3C; 4F– 4G; 5B; (map)) |
| Haematodes tenuipes Kraatz, 1858: 363 |
| Weiserianum woltersi Bernhauer, 1927: 248 syn. nov. |
Type locality. ‘Brazil’.
Type material. Haematodes tenuipes Kraatz. Lectotype (male, DEI) (here designated): Brasil [handwritten, green label] / Syntypus [printed red label] / DEI Müncheberg Col –09387 [printed green label] / Haematodes tenuipes Kr. [written white label] / Haematodes tenuipes LECTOTYPE des. Brunke and Chatzimanolis 2018 [printed red label].
The studied and dissected male syntype deposited in SDEI was selected and is here designated as a lectotype of Haematodes tenuipes Kraatz, 1858. We designated a lectotype to stabilize the nomenclature of this taxon, which is morphologically similar to H. myteros.
Weiserianum woltersi Bernhauer, 1927 syn. nov. Holotype (female, FMNH): Corral Quemado, Catam. Wolters [handwritten label] / Weiserianum woltersi Typ, Bernh., Typus un., Bruch. [handwritten label] / Chicago NHMus, M. Bernhauer Collection [typed white label] / Weiserianum woltersi HOLOTYPE det. Brunke and Chatzimanolis 2018 [printed red label] / Haematodes tenuipes Kraatz det. Brunke and Chatzimanolis 2018 [printed white label].
The female holotype of W. woltersi is from ‘northwest Argentina, Catamarca, Corral Quemado’, 2,000 m in the Andes. This is currently a long distance from the other known specimens of H. tenuipes but this species is likely under-collected and H. bicolor has a comparably broad distribution. However, there is a possibility of mislabeling and further collecting will hopefully clarify this issue. Externally, this specimen falls within the range of variability for H. tenuipes.
Other material. BRAZIL: ‘Brasil’, 1940, P. Grossa (1, CNC); Paraná: Curitiba, 15.x.1955, C.D. Michener, SM0046430 (1, SEMC); Rio Grande do Sul: Santo Augusto, X.1966, O. Roppa (7, CNC); Santa Catarina: Nova Teutônia, 27°11′S 52°23′W, 3.X.1937, F. Plaumann (1, ZMHB). São Paulo: Campinas, Mogi-Guaçu, 1–8.I.1970, J.M. & B.A. Campbell (1, CNC).
Diagnosis. This species can be recognized by a combination of: pronotum with at least a few discal punctures (Fig. 3C); elytra reddish (Fig. 1C); and pronotum transverse (Fig. 3C).
Redescription. Forebody length 7.04 –8.24 mm. Body pale reddish orange to yellow-orange, antenna and legs brown, scutellum, lateral margin of pronotum and abdominal segments III–VI black.
Head transverse, temples moderately expanded behind eyes, nuchal ridge present, either entire or erased at middle, width/length ratio = 1.48–1.66, widest just anteriad of neck, dorsal surface moderately densely punctate, with variably-sized glabrous area at middle; eyes nearly half of head length. Antennae short, segments 4–10 strongly transverse and forming an elongate club (Fig. 2B), segments 5–10 slightly asymmetrical, tomentose pubescence missing medially on segments 5–10, segments 1–3 with bristle-like setae latero-apically (Fig. 2C), apical segment weakly to moderately transverse, and with weakly emarginate apex. Legs with tibia expanded and elongate triangular; hind tarsi slightly broadened and slightly flattened (Fig. 2F). Pronotum width/head width ratio = 1.09–1.20, moderately transverse (Fig. 3C), width/length ratio = 1.22–1.34; disc of pronotum with punctures arranged into dorsal and sublateral longitudinal rows, punctures usually doubled (Fig. 3C), anterior angles and lateral margins with long and bristle-like setae (Fig. 3C); punctation of elytra and abdomen sparse, with apical half of tergite VII and VIII glabrous (Fig. 1C).
Male sternite VII with porose structure as in Fig. 2I, relatively thin, occupying less of the sternal width than H. kuntzeni. Male sternite VIII with shallow emargination. Aedeagus as in Figs. 4F, 4G. In lateral view, median lobe with two approximate subapical teeth and a relatively short, acuminate apical portion that is sharply constricted near apex (Fig. 4F). Paramere with apex weakly trilobed and with two groups of peg setae, distinctly fusing apically (Fig. 4G).
Distribution. This species is currently known from Catamarca province in Argentina, and the states of Paraná, Rio Grande do Sul, Santa Catarina and São Paulo in Brazil (Fig. 5B).
Bionomics. Nothing is known about this species’ biology.
Type locality. San Rafael Reserve, Cazaapá, Paraguay.
Type material. Holotype (male, SEMC): PARAGUAY: Cazaapá, Estancia Condesa/Toro Blanco, San Rafael Reserve, 110m, 26° 19′11′S, 55° 39′57′W, 8–10 DEC 2000, Z. H. Falin, PAR1F00 140 ex. flight intercept trap [printed label] / SM0235421 [barcode printed label] / Haematodes sp. det. J.S. Ashe 2001 [printed label] / HOLOTYPE Haematodes myteros Brunke and Chatzimanolis, des. Brunke and Chatzimanolis 2018 [red printed label]. In the collection of SEMC.
Paratypes (1 male, NMNH; 1 f#, ZMHB): Dept. Agr. Nac. Caacupe, PARAGUAY, Depto. Cordillera, 12.II.1981 [printed label] / R.D. Cave colr. [printed label] / PARATYPE Haematodes myteros Brunke and Chatzimanolis, des. Brunke and Chatzimanolis 2018 [yellow printed label]. In the collection of NMNH.
Brasilien, Nova Teutonia, 27°11′S 52°23′W, 4.IX.1934, F. Plaumann [printed label] / Haematodes bicolor Laporte, Museum f. Naturkunde, Berlin [white printed label] / PARATYPE Haematodes myteros Brunke and Chatzimanolis, des. Brunke and Chatzimanolis 2018 [yellow printed label]. In the collection of ZMHB.
Diagnosis. This species can be recognized by a combination of: pronotum with at least a few discal punctures (Fig. 3D); elytra reddish (as in Fig. 1C); and pronotum subquadrate (Fig. 3D).
Description. Extremely similar in morphology to H. tenuipes and differing only in the following: forebody length 6.20–7.61 mm; head width/length ratio = 1.45–1.57. Pronotum width/head width ratio = 1.11–1.27, width/length ratio = 1.11–1.17, subquadrate (Fig. 3D); disc of pronotum with punctures arranged into dorsal and sublateral longitudinal rows, punctures not doubled (Fig. 3D); aedeagus as in Figs. 4H–4I; in lateral view, median lobe with two approximate subapical teeth and a relatively elongate apical portion that is not constricted near apex (Fig. 4H). Paramere with apex emarginate and with two groups of peg setae, distinctly fusing apically (Fig. 4G).
Distribution. Currently known from Cordillera and Cazaapá departments in Paraguay, and Santa Catarina state in Brazil (Fig. 5B).
Bionomics. The holotype was collected in a flight intercept trap in San Rafael Reserve.
Etymology. The species epithet is the Greek word meaning ‘pointed’ and refers to the median lobe in this species, which is thinner and more acute in lateral view than that of the other Haematodes.