Mesopredatory fishes from the subtropical upwelling region off NW-Africa characterised by their parasite fauna
- Published
- Accepted
- Received
- Academic Editor
- Jean-Lou Justine
- Subject Areas
- Ecology, Marine Biology, Parasitology, Zoology
- Keywords
- Food-web, Nealotus tripes, Subtropical East-Atlantic, Trichiurus lepturus, Eastern boundary upwelling ecosystem, Canary Current
- Copyright
- © 2018 Alt et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2018. Mesopredatory fishes from the subtropical upwelling region off NW-Africa characterised by their parasite fauna. PeerJ 6:e5339 https://doi.org/10.7717/peerj.5339
Abstract
Eastern boundary upwelling provides the conditions for high marine productivity in the Canary Current System off NW-Africa. Despite its considerable importance to fisheries, knowledge on this marine ecosystem is only limited. Here, parasites were used as indicators to gain insight into the host ecology and food web of two pelagic fish species, the commercially important species Trichiurus lepturus Linnaeus, 1758, and Nealotus tripes Johnson, 1865. Fish specimens of T. lepturus (n = 104) and N. tripes (n = 91), sampled from the Canary Current System off the Senegalese coast and Cape Verde Islands, were examined, collecting data on their biometrics, diet and parasitisation. In this study, the first parasitological data on N. tripes are presented. T. lepturus mainly preyed on small pelagic Crustacea and the diet of N. tripes was dominated by small mesopelagic Teleostei. Both host species were infested by mostly generalist parasites. The parasite fauna of T. lepturus consisted of at least nine different species belonging to six taxonomic groups, with a less diverse fauna of ectoparasites and cestodes in comparison to studies in other coastal ecosystems (Brazil Current and Kuriosho Current). The zoonotic nematode Anisakis pegreffii occurred in 23% of the samples and could pose a risk regarding food safety. The parasite fauna of N. tripes was composed of at least thirteen species from seven different taxonomic groups. Its most common parasites were digenean ovigerous metacercariae, larval cestodes and a monogenean species (Diclidophoridae). The observed patterns of parasitisation in both host species indicate their trophic relationships and are typical for mesopredators from the subtropical epi- and mesopelagic. The parasite fauna, containing few dominant species with a high abundance, represents the typical species composition of an eastern boundary upwelling ecosystem.
Introduction
Marine productivity is particularly high off the West African coast due to strong seasonal upwelling processes (Van Camp et al., 1991; Mbaye et al., 2015). The upwelling in the north-eastern Atlantic tropical upwelling system is mainly wind-driven and enhanced by the Gyre of Guinea in summer (Faye et al., 2015). This eastern boundary upwelling ecosystem (EBUE) is an important resource for fisheries (FAO fishing area 34, Eastern Central Atlantic), with 2.5% of annual catches worldwide originating from this region (FAO, 2012). Despite the economic relevance of West African fishery resources as well as records of overexploitation of small fish species, e.g., Scomber japonicus and Engraulis encrasicolus, data on commercially relevant fish species is scarce (FAO, 2009; Meissa, Gascuel & Rivot, 2013).
A fish species frequently encountered in the north-eastern Atlantic tropical upwelling region is the cutlassfish Trichiurus lepturus Linnaeus, 1758 (Trichiuridae). It inhabits the continental shelves to 350 m depths and can be found throughout temperate and tropical regions worldwide. Cutlassfish is a highly relevant commercial fishery resource with stable annual captures of 1.3 million tons since 2008 (Nakamura & Parin, 1993; FAO, 2014). As one of the ten most important species targeted by marine fisheries worldwide, its annual take is similar to other commercially important species, e.g., yellowfin tuna (Thunnus albacares) and Atlantic cod (Gadus morhua) (FAO, 2014). Cutlassfish is a popular food fish especially in Asia and usually cooked or served raw as sashimi (Nakamura & Parin, 1993).
Another fish species found in the same tropical upwelling region is the black snake mackerel Nealotus tripes, Johnson 1865 (Gempylidae). N. tripes can be found in mesopelagic habitats at depths to 600 m (Nakamura & Parin, 1993), with a global distribution in tropical and temperate waters (Strasburg, 1964; Yatsu et al., 2005; Tanaka, Mohri & Yamada, 2007; Mafalda Jr, Souza & Weiss, 2009; De Forest & Drazen, 2009; Wienerroither et al., 2009; Ivanov & Sukhanov, 2015). As a nyctoepipelagic species, it roams mesopelagic regions at daytime and migrates to the surface at night (e.g., Boehlert, Watson & Sun, 1992; Yatsu et al., 2005). Most gempylid species are not of commercial interest, except Thyrsites atun and Rexea solandri (Nakamura & Parin, 1993).
Marine ecosystems contain a broad diversity of parasites, which can serve as ecological indicators. Thus, parasites can be used as indicators for their host, reveal anthropogenic impact, e.g., accumulation of contaminants, or show systemic influences (Wood, 2007; Palm & Rückert, 2009; Palm, 2011; Wood et al., 2013; Wood et al., 2014). Moreover, the biodiversity of parasites in an ecosystem reflects the diversity of host organisms throughout its food web (Klimpel, Seehagen & Palm, 2003; Rückert, Palm & Klimpel, 2008). Combined with stomach content analyses of the hosts, parasitological data can therefore provide important insights into food webs, host-parasite relationship and host ecology in marine ecosystems. The parasite fauna and food ecology of T. lepturus have been studied before, however, there is a lack of data in the Canary Current System (CCS) (Timi, Martorelli & Sardella, 1999; Martins & Haimovici, 2000; Ho & Lin, 2002; Shih, 2004; Martins, Haimovici & Palacios, 2005; Bryan & Gill, 2007; Palm & Klimpel, 2007; Carvalho & Luque, 2011; Carvalho & Luque, 2012; Bueno, Aguiar & Santos, 2014). No data on the parasite fauna of N. tripes have been collected so far.
To gain insights into the CCS, the diet-composition and parasite fauna of T. lepturus and N. tripes from different habitats off Senegal and Mauritania were examined. The overall aim of this study was to assess whether the parasite fauna of pelagic fishes in the CCS represents the low species richness typically known from EBUEs (Angel, 1993; Sakko, 1998). Data from the commercially important Trichiurus lepturus were compared to findings from other studies in different coastal ecosystems.
Material and Methods
The samples of T. lepturus and N. tripes were taken during the 375th cruise of the research vessel Walther Herwig III. Sampling took place in the tropical East-Atlantic (FAO fishing area 34, 3.11) at different sampling sites off the North-West-African coast (Fig. 1). The catch was yielded by trawl fishing with a multisampler. After landing, the catch was sorted, identified to species level and weighed. The samples were stored in a freezer at −20 °C and defrosted over night at 4 °C in a refrigerator or 90 min at room temperature before examination. Specimens of T. lepturus (n = 104) and N. tripes (n = 91) were examined by assessing biometrical measures, stomach contents and parasite fauna of the fishes.
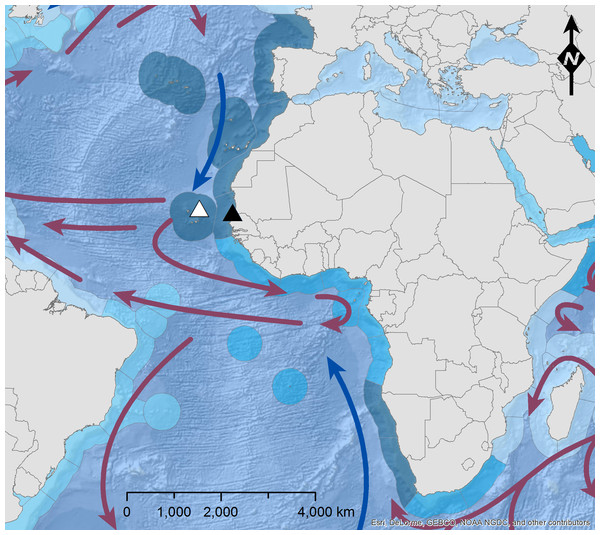
Figure 1: Map of the sampling area.
Sampling area of T. lepturus (black triangle; haul from N16°45.49′, W16°38.16′to N16°43.34′, W16°39.76′) and N. tripes (white triangle; haul from N17°30.93′, W22°57.29′to N17°31.48′, W22°58.24′) off NW-Africa, with major ocean currents (blue arrow = cold; red arrow = warm; NOAA, National Weather Service, Maps.com) and coastal upwelling activity (increasing from light to dark blue; Hoekstra et al., 2010). The figure was built using ESRI ArcGIS 10.3 (ESRI, Redlands, CA, USA).The stomach of each specimen was weighed full and empty to assess the total weight of its content. The different food components were sorted to the lowest possible taxonomic level, counted and weighed. Depending on the degree of digestion, prey numbers were identified by counting eyepairs (Crustacea), otoliths, vertebrae (Teleostei) and beaks (Cephalopoda). Trophic measures were calculated according to Hyslop (1980) for all specimens with stomachs containing food.
The body surface, fins, buccal cavity, nasal cavities and gills of the fishes were inspected for ectoparasites using a stereo microscope. Subsequently, the inner organs were examined for endoparasites. Parasites were stored in 99% ethanol for molecular identification or preserved in Roti®Histofix 4% (Carl Roth GmbH, Karlsruhe) for morphological examination and then cleared in glycerine and mounted on microscope slides. Parasitological measures were determined for each parasite taxon according to Bush et al. (1997).
Nematode larvae and myxozoans were identified by molecular methods. The DNA extraction from parasites was performed using a kit (PeqGOLD Microspin Tissue DNA Kit Protocol for small tissue sizes; Peqlab, VWR International GmbH, Darmstadt, Germany) and Acroprep plate (Pall Corporation, Port Washington, NY, USA). DNA samples were stored at 4 °C and processed promptly.
For the identification of the nematodes, the internal transcribed spacers and 5.8S rDNA were amplified, using primers TK1 (Anisakis spp.), NC5 (Hysterothylacium spp.) and NC2 (Zhu et al., 2000; Kuhn, García-Màrquez & Klimpel, 2011). A thermal cycler (Mastercycler, nexus gradient (eco); Eppendorf AG, Hamburg, Germany) was programmed to perform six steps: 1) heating (95 °C, 1 min), (2) denaturation (94 °C, 45 s), (3) annealing (55 °C, 45 s), (4) elongation (72 °C, 45 s), (5) (72 °C, 10 min) and (6) cooling (4 °C, ∞ min). Steps 2 to 4 were repeated in 40 cycles. PCR products were stored at 4 °C and processed promptly.
Myxozoan parasites were identified to species level using the 18S rDNA marker according to Shin et al. (2016) applying the primers Kudo-ShinF and Kudo-ShinR and the PCR-conditions described therein. A pairwise alignment was performed using the nucleotide Basic Local Alignment Search Tool (nBLAST, National Center for Biotechnology Information), searching NCBI Genbank for matches (Altschul et al., 1990).
A list of the parasites of Trichiurus lepturus was compiled by running a search on ‘Google-Scholar’ and ‘ISI-Web of Knowledge’ with the keyword combination “Trichiurus parasit*”. In addition, the Host-Parasite-Database of the National History Museum, London was checked for entries of T. lepturus and its synonym T. haumela as a host (Gibson, Bray & Harris, 2005). The taxon validity of the parasite records was checked using the World Register of Marine Species (WoRMS Editorial Board, 2017). There is no claim that the resulting list is comprehensive.
Results
Morphometrical data
Of the 104 examined Trichiurus lepturus, 59 individuals were male, 44 were female, and all gonads were premature. The mean total length (TL) of T. lepturus was 60.3 cm (±6.4 cm SD). The preanal length (PL) was approximately one third of the TL, with a median at 20.8 cm. The median total weight (TW) was 128.1 g and the median carcass weight (CW) was 120.3 g.
The sample of N. tripes (n = 91) was composed of 32 male and 58 female individuals. The median total length was 18.0 cm and the median preanal length was 11.1 cm. The median TW was 23.3 g and the median CW was 20.3 g. Detailed morphometric data are given in Table S1.
Food ecology
Stomach content analyses revealed prey organisms from three taxonomic groups in both fish species, namely Mollusca, Crustacea and Teleostei (Tables 1 and 2).
| Prey item | n | F [%] | W [%] | N [%] | IRI |
|---|---|---|---|---|---|
| Mollusca | 8 | 7.7 | 2.67 | 0.020 | 20.99 |
| Cephalopoda spp. | 8 | 7.7 | 2.67 | 0.020 | 20.99 |
| Crustacea | 95 | 92.2 | 44.17 | 99.683 | 13,268.62 |
| Decapoda spp. | 2 | 0.9 | 0.04 | 0.002 | 0.05 |
| Mysida spp. | 95 | 92.2 | 44.12 | 99.660 | 13,261.90 |
| Teleostei | 54 | 52.4 | 53.20 | 0.310 | 2,805.41 |
| Clupeiformes spp. | 31 | 13.5 | 40.39 | 0.092 | 550.25 |
| Trichiuridae sp. | 2 | 1.9 | 0.2 | 0.005 | 0.54 |
| indet. | 38 | 52.4 | 12.5 | 0.211 | 470.29 |
| Prey item | n | F [%] | W [%] | N [%] | IRI |
|---|---|---|---|---|---|
| Mollusca | 2 | 2.2 | 0.5 | 1.66 | 4.83 |
| Cephalopoda spp. | 2 | 2.2 | 0.5 | 1.66 | 4.83 |
| Crustacea | 5 | 5.5 | 0.5 | 4.1 | 26.22 |
| Decapoda spp. | 4 | 3.3 | 0.5 | 3.33 | 17.27 |
| Amphipoda sp. | 1 | 1.1 | 0.001 | 0.83 | 0.92 |
| Teleostei | 90 | 100.0 | 98.9 | 94.17 | 19,310.32 |
| Myctophidae spp. | 15 | 16.6 | 31.6 | 14.16 | 764.05 |
| indet. | 75 | 83.3 | 67.9 | 84.95 | 15,293.88 |
The main diet organisms of T. lepturus were small, pelagic Crustacea. With a frequency of occurrence of 92.2% and a numerical percentage of prey of 99.6% Mysida were the most important food item of T. lepturus (IRI = 13,261.9). Fish prey occurred with a frequency of 52.4%, including 1.9% of possible cannibalism (Trichiuridae). Small bony fish had the highest weight percentage of prey (W = 53.2%), followed by the Mysida (W = 44.12%). Cephalopoda and Decapoda were rare food items in specimens examined in this study.
The main food items of N. tripes were small bony fish. In all examined stomachs containing food (90/91), small fish were identified. Fish was found to be the most important food item of the specimens examined in this study, by frequency (F = 100%), weight percentage (W = 98.9%) and numerical percentage (N = 94.17%). While a large proportion of fish prey was in an advanced stage of digestion, 17 prey fishes were identified as Myctophidae. Five examined stomachs contained Crustacea, a single amphipod and four decapods, and two stomachs contained Cephalopoda in advanced stages of digestion.
Parasite fauna
Previous studies have identified more than 50 parasite taxa in six taxonomic groups parasitising T. lepturus. Most records are of digenetic trematodes, followed by nematodes (Table S2). In this study, the parasite fauna of T. lepturus consisted of at least nine different species belonging to six taxonomic groups (Table 3). The most frequent parasite was the digenean Lecithochirium microstomum, with a prevalence of 88.4% (mI = 58.6) in the stomach and rarely pyloric caeca. Ovigerous metacercarieae of a second digenean were less common and occurred with a prevalence of 7.6% (mI = 1.5) encapsulated in the body cavity and mesenteries. The intensity of digenean infection was positively correlated with the total length of the hosts (Spearman r = 0.72, p < 0.001). The parasite group with the highest intensity and abundance was the unidentified, early larval stages of cestodes. Unidentified tetraphyllidean cestode larvae occurred in the intestine and pylorus of 40.3% of the examined fish (mI = 19.6). Smaller fish were infested with higher intensities of cestode larvae than larger individuals (Spearman r = − 0.39, p < 0.001). At least three nematode species of the families Anisakidae and Raphidascarididae were isolated from the body cavity and liver surface and identified molecularly. Anisakis pegreffii (P = 23%) and A. typica (P = 17.3%) occurred with similar prevalences and low intensities (I = 1–2 for both), while Hysterothylacium sp. was less frequent (P = 2.8%). Higher infection intensities (I = 1–4) were shown for very small, unidentified nematode larvae, that were encapsulated in the stomach tissue. The pylorus of one specimen of T. lepturus was infested with a single acanthocephalan.
| Parasite taxon | site | stage | P [%] | I | mI | mA |
|---|---|---|---|---|---|---|
| Digenea | ||||||
| Lecithochirium microstomum | s | A | 88.4 | 1–222 | 58.6 | 51.85 |
| indet. | bc, m | L | 7.6 | 1–5 | 2.0 | 0.15 |
| Monogenea | ||||||
| Octoplectanocotyla travassosi | g | A | 12.5 | 1–6 | 1.5 | 0.19 |
| Cestoda | ||||||
| Tetraphyllidea indet. | i | L | 52.8 | 1–∼5,000 | >900 | >400 |
| Nematoda | ||||||
| Anisakis pegreffii | bc | L | 23.0 | 1–2 | 2.0 | 0.26 |
| Anisakis typica | bc | L | 17.3 | 1–2 | 1.1 | 0.25 |
| Hysterothylacium sp. | p, i | L | 2.8 | 1 | 1.0 | 0.02 |
| indet. | i, sc | L | 25.0 | 1–14 | 2.0 | 0.5 |
| Acanthocephala | ||||||
| indet. | i | 1.9 | 1 | 1.0 | 0.01 | |
| Crustacea | ||||||
| Bomolochidae indet. | n | A | 0.9 | 1 | 1 | <0.01 |
| Caligidae indet. | g | A | 5.7 | 1–2 | 1.1 | 0.06 |
Notes:
- bc
-
body cavity
- g
-
gills
- gb
-
gall bladder
- i
-
intestine
- l
-
liver
- m
-
mesentery
- p
-
pylorus
- s
-
stomach
- sc
-
stomach capsule
- A
-
adult
- L
-
larval
The only ectoparasites identified in this study were isolated from the gills. The monogenean Octoplectanocotyla travassosi occurred with a prevalence of 12.5% in T. lepturus, while Copepoda were present with a prevalence of 6.7%.
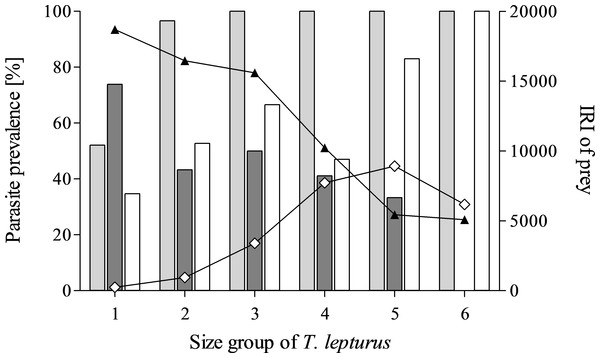
The diet composition of T. lepturus depended on its size. In larger specimens, the IRI of crustacean prey items decreased, while the IRI of teleost prey increased. The prevalence of the most important parasite taxa also varied between size groups of the fish host with increasing size, the prevalences of cestode larvae decreased, while digeneans and nematodes increased (Fig. 2).
Figure 2: Parasite prevalence and prey importance in the size groups of T. lepturus.
Prevalence of Digenea (light grey), Cestoda (dark grey) and Nematoda (white) and Index of Relative Importance (IRI) of Crustacea (black triangle) and Teleostei (white diamond) of different size groups of T. lepturus. Size group 1–6; <50 < 55 < 60 < 65 < 70 < 75 cm.The parasite fauna of N. tripes was composed of at least thirteen species from seven different taxonomic groups (Table 4). The muscle tissue of one specimen was infected with the myxozoan Kudoa thyrsites. The parasite was detected through a noticeable softening of the host muscle tissue. Digenean ovigerous metacercariae were the parasites with the highest prevalence in N. tripes (P = 62.9%) and occurred free or encysted with an intensity range between one and 82 parasites per host. Adult stages of this parasite were isolated from the body cavity, mesenteries and blood vessels. Digeneans from the family Hemiuridae were identified in two other fish specimens, encysted and degraded.
| Parasite taxon | Site | Stage | P [%] | I | mI | mA |
|---|---|---|---|---|---|---|
| Myxozoa | ||||||
| Kudoa thyrsites | mu | 1.0 | – | – | – | |
| Digenea | ||||||
| indet. | bc, m, gb | L | 69.2 | 1–82 | 9.6 | 6.64 |
| Hemiuridae indet. | A | 2.1 | 1 | 1 | 0.02 | |
| Monogenea | ||||||
| Diclodophoridae indet. | g | A | 58.2 | 1-7 | 1.5 | 0.92 |
| Cestoda | ||||||
| Tetraphyllidea indet. | p, gb, bd | L | 58.2 | 1–235 | 55.6 | 32.3 |
| indet. | L | 42.8 | 1->9,000 | 907.1 | 220.40 | |
| Nybelinia sp. | sc | L | 6.5 | 1–2 | 1.1 | 0.07 |
| Trypanorhyncha spp. | 3.2 | 1–2 | 1.3 | 0.04 | ||
| Nematoda | ||||||
| Anisakis physeteris | L | 3.2 | 1–2 | 1.3 | 0.04 | |
| Anisakis typica | bc | L | 15.3 | 1–2 | 1.0 | 0.16 |
| Anisakis sp. | L | 4.3 | 1–2 | 1.2 | 0.05 | |
| Hysterothylacium sp. | i | L | 1.0 | 1 | 1.0 | 0.01 |
| indet. | i, sc | L | 20.8 | 1–35 | 3.8 | 0.81 |
| Acanthocephala | ||||||
| indet. | i | 6.5 | 1 | 1.0 | 0.07 | |
| Crustacea | ||||||
| Bomolochidae indet. | g | A | 25.2 | 1–2 | 1.3 | 0.36 |
| Lernaeenicus sp. | e | A | 1.0 | 1 | 1.0 | 0.01 |
Notes:
- bc
-
body cavity
- bd
-
bile ducts
- e
-
eye
- g
-
gills
- gb
-
gall bladder
- i
-
intestine
- l
-
liver
- m
-
mesentery
- mu
-
muscle
- p
-
pylorus
- s
-
stomach
- sc
-
stomach cyst
- A
-
adult
- L
-
larval
Cestode larvae were the most abundant parasite group. Larvae isolated from the pylorus and intestine occurred with intensities up to 9,000 (estimate) (P = 24.1%). Unidentified tetraphyllidean cestode larvae were isolated from the pylorus, bile ducts and gall bladder. Cestodes from the family Trypanorhyncha were less common. Larval stages from the genus Nybelinia were isolated from cysts in the stomach wall (P = 6.5%). Nematodes from the families Anisakidae and Raphidascarididae were isolated from the body cavity of N. tripes. A. typica occurred with a prevalence of 15.3%. Furthermore, A. physeteris, Anisakis sp. and Hysterothylacium sp. were molecularly identified. Intensity of these nematode larvae ranged from one to two parasites per host. Minuscule larvae were isolated from cysts in the stomach wall (P = 20.8%).
Ectoparasites were isolated from the mouth and gills of N. tripes. Monogeneans from the family Diclidophoridae occurred with a prevalence of 58.2%. Parasitic crustaceans from the family Bomolochidae were less frequent (P = 25.2%).
Discussion
Species richness in EBUEs is considered to be low in comparison to other tropical marine ecosystems (Angel, 1993; Sakko, 1998). Strong seasonal upwelling activities and circulation of cold, nutrient rich water do not only require adaption of the host organisms (e.g., fish spawning and recruitment), but also the parasite fauna (Carvalho & Luque, 2011; Cropper, Hanna & Bigg, 2014; Sambe et al., 2016; Tiedemann & Brehmer, 2017; Tiedemann et al., 2017). As a consequence, the parasite diversity found in EBUEs should be lower than in other coastal ecosystems. In the following paragraphs the parasite fauna as well as results of the stomach content analyses will be discussed separately for each of the two fish species from the CCS and, in the case of Trichiurus lepturus, compared with other studies from different coastal ecosystems (Table S2).
Trichiurus lepturus
The parasite fauna of T. lepturus observed in this study was less diverse than in previous studies from other coastal regions in the Brazil Current and Kuroshio Current. Digeneans of the family Hemiuridae were the taxonomic group infesting T. lepturus with the highest prevalence. The diversity of digeneans described throughout the literature has a geographical pattern. There is a sampling bias towards studies from the Chinese Sea, probably due to the importance of T. lepturus as a food fish. In these studies, most species were described (Table S2). The species L. microstomum was the predominant parasite of T. lepturus in this study (P = 88.4%). A high mean intensity (mI = 58.61) reflects an aggregated occurrence of L. microstomum. Similar prevalences have been reported in T. lepturus sampled from coastal locations off Brazil (Carvalho & Luque, 2011; Bellay et al., 2011; Bueno, Aguiar & Santos, 2014). Bellay et al. (2011) characterised L. microstomum as a network hub species that is highly generalistic and parasitic in unrelated definitive host species. Such parasite species can be expected to thrive in EBUEs. L. microstomum has been described from several small fish species, e.g., Engraulis anchoita, which are typical prey organisms of adult T. lepturus (Timi, Martorelli & Sardella, 1999; Martins, Haimovici & Palacios, 2005). In this study, the prevalence and intensity of L. microstomum was shown to increase with the size of the host, while teleost prey gains importance (Fig. 2). Bueno, Aguiar & Santos (2014) described an ontogenetic shift in the diet of T. lepturus, from krill to fish, which is also supported by our data.
Cestode larvae occurred with high intensities of between one and >5,000 parasites per host individual. The relationship between the size of the fish and the intensity of cestode infection implies that smaller fish are more susceptible to higher infection intensities. This is likely influenced by either an ontogenetic change in its diet composition, or by the development of a host immune-response, or a combination of these factors (e.g., Stunkard, 1977; Marcogliese, 1995; Buchmann, 2012). This hypothesis is also supported by the findings of previous studies examining larger specimens, where reported infection intensities were much lower and the cestode larvae were further developed (Shih, 2004; Carvalho & Luque, 2011; Bueno, Aguiar & Santos, 2014). It is also possible that the density of cestode larvae varies according to the upwelling seasonality (Carvalho & Luque, 2011). In order to examine this hypothesis, samples need to be collected during and between recurring upwelling events. Taking previous research into account, a higher diversity of cestode larvae would have been expected in T. lepturus (Table S2) (Shih, 2004; Carvalho & Luque, 2011; Bueno, Aguiar & Santos, 2014).
Trichiurus lepturus has been described as host organism of various nematodes (Table S2) and serves as an intermediate host for nematodes identified in the present study. As the data in this study show, T. lepturus could accumulate anisakid nematode larvae with increasing size and age, when fishes gain importance as prey, and then serve as a paratenic host (Martins, Haimovici & Palacios, 2005). Anisakis spp. have a pelagic life cycle with Crustacea as first intermediate hosts, matching the epipelagic feeding behaviour of T. lepturus, which feeds on large batches of euphausiids and small fish (Nakamura & Parin, 1993; Abollo, Gestal & Pascual, 2001; Mattiucci & Nascetti, 2006). A. pegreffii is closely related to A. simplex (s.s.) and commonly found in mid-Atlantic waters and the Mediterranean (Klimpel et al., 2010; Mattiucci et al., 2013). Fish specimens in the present study were caught considerably further south than the southern limit of A. simplex (s.s.) (Kuhn et al., 2013). Implied by the absence of A. simplex (s.s.) in the two fish hosts, the fish from the present sample did not migrate further north than Gibraltar. Kong et al. (2015) identified A. pegreffii with a prevalence of 84.4% in adult T. lepturus from Chinese waters and Anisakis simplex (s.s.) and A. typica were detected with lower prevalences of 0.6% and 1.5% respectively. In comparison, the prevalence of A. typica was high in the present study Borges et al. (2012) found higher prevalences of A. typica (20.3%) and Hysterothylacium sp. (51.3%) in the Brazil Current region. Another study from this region recorded prevalences of anisakid nematode larvae in T. lepturus ranging from 73.3 to 100% throughout the seasons (Carvalho & Luque, 2011). The different prevalences of Anisakis spp. can be explained by a location effect and the availability of the definitive hosts (toothed whales, mainly delphinids), e.g., coastal dwelling dolphins for A. typica (Mattiucci & Nascetti, 2008). As Hysterothylacium is transmitted to fish through (crustacean) invertebrate hosts, the higher prevalence found by Borges et al. (2012) may be connected to the feeding habits of the fish. Also, Køie (1993) showed that the size of the larvae of H. aduncum during ingestion of the crustacean host by a fish might be crucial to the parasite’s survival.
The nematode fauna of T. lepturus has a zoonotic potential because of the occurrence of A. pegreffii, which may cause anisakiasis. An infection may go along with gastro-intestinal or allergic symptoms (Zhu et al., 1998; Daschner & Pascual, 2005; Nieuwenhuizen et al., 2006; Hochberg & Hamer, 2010; Mattiucci et al., 2013). A. pegreffii is the main cause of anisakiasis in the Mediterranean (Mattiucci et al., 2013). If located on the visceral surface of the intestine, migration of the parasite into the muscle tissue of the fillet might be favoured, resulting in a possible health hazard (Klapper et al., 2015; Mladineo et al., 2017). However, the risk of ingesting a living larva from a correctly gutted fish can be considered as rather small. This is due to the body shape of T. lepturus and the position of its body cavity which only expands over a third of the total length of the fish. Nematode larvae can migrate into the muscle but are mostly found in the belly flaps of the fish, which are usually not prepared as a food (Klapper et al., 2015). To assess whether nematodes migrate into the muscle tissue of T. lepturus, appropriate techniques e.g., pepsin-HCl-digestion should be applied. The presence of the larvae in the visceral tissue of T. lepturus can be conceived as potentially hazardous with respect to food allergies in sensitized individuals (Nieuwenhuizen et al., 2006; Mattiucci et al., 2013).
The infestation with ectoparasites is most likely due to the schooling behaviour of subadult T. lepturus, which provides good conditions for the transmission of monoxenic monogeneans and Copepoda (Hunter, 1966; Carvalho & Luque, 2011; Johnson et al., 2011). In comparison to other studies, ectoparasites were scarce (Carvalho & Luque, 2011; Carvalho & Luque, 2012; Bueno, Aguiar & Santos, 2014). Trichiurus lepturus sampled from the warm Brazil Current, which has a rather moderate upwelling activity, had a higher diversity of ectoparasites (three monogenean species, two copepod species) than the specimens from the Canary Current examined in this study (one monogenean species, one copepod species) (Bueno, Aguiar & Santos, 2014). From this finding we conclude that the intense seasonal upwelling activity in the CCS-EBUE might diminish parasite diversity and promote generalist species.
N. tripes
The newly recorded parasite fauna of N. tripes was dominated by three taxa, digenean larvae (mostly encysted ovigerous metacercariae), tetraphyllidean cestode larvae and a monogenean species from the family Diclidophoridae. The presence of digenean larvae suggests benthic feeding behaviour, because gastropods are obligatory intermediate hosts. As a pelagic species with a diet composed of a large proportion of teleost prey, their route of infection for N. tripes is most likely through a teleost transport host. Previous studies have shown, that preadult stages of the genus Lecithochirium occur encysted in the viscera of the host (Gibson & Bray, 1986). Free stages in the intestine are possibly transitioning to the viscera, where they might encapsulate (Gibson & Bray, 1986). Thus, the digenean trematodes extracted from our specimens could belong to this genus.
The cestodes isolated from N. tripes provide a similar picture to that in T. lepturus. High intensities of small unidentifiable larvae were isolated from the digestive system. The larval tetraphyllidean cestode morphotaxon Scolex pleuronectis was previously recorded, but no peer-reviewed parasitological studies on N. tripes have been published. The bile ducts and gall bladder as sites of infection for tetraphyllidean cestode larvae have been observed in other fish species (e.g., Hippoglossus stenolepis, Blaylock, Holmes & Margolis, 1998) and are identified in N. tripes for the first time. Many aspects of the life-cycle of tetraphyllidean cestodes are still unknown, but most species have elasmobranch definitive hosts (Marcogliese, 1995). This indicates that T. lepturus and N. tripes may be important prey organisms for pelagic sharks in this area. In contrast to T. lepturus, no connection between the cestode infection intensity and the length and weight of the fish could be made. A possible reason for the high abundance of cestode larvae is the piscivorous diet. A parasite fauna of larval cestodes and nematodes and low species richness has been described as the typical parasite fauna of myctophid fishes, the main diet component of N. tripes (Klimpel, Kellermanns & Palm, 2008). Myctophids play an important role in the transmission of parasites throughout the water column, especially nematodes from the genus Anisakis (Klimpel, Kellermanns & Palm, 2008). Mateu et al. (2015) described Myctophidae as a link in the life-cycle of A. physeteris, connecting the crustacean host to the squid, which are consumed by the definitive host, the sperm whale Physeter macrocephalus (Mattiucci & Nascetti, 2008; Mateu et al., 2015 and the references therein). It seems that N. tripes plays a similar role as Myctophidae, as they also occupy an intermediate position in the food web (linking zooplankton to large vertebrates) and perform diurnal vertical migrations (Boehlert, Watson & Sun, 1992; Yatsu et al., 2005). The presence of A. physeteris and A. typica in our sample is an indicator of this, as the former is host specific to P. macrocephalus, which inhabits deeper water layers and the latter is host specific to dolphins that prefer the epipelagic (Mattiucci & Nascetti, 2008; Mateu et al., 2015). It also indicates that N. tripes might be preyed upon by squid.
The monogenean infestation of N. tripes is an indicator of schooling-behaviour. Many monogenean species are host specific, their adhesive organs (haptoral clamps) being adapted to the gills of the host (Boeger & Kritsky, 1993). Thus, it is possible that the present diclidophorid parasite species has not yet been described. N. tripes is a new host record for K. thyrsites, A. typica and A. physeteris.
Parasite richness
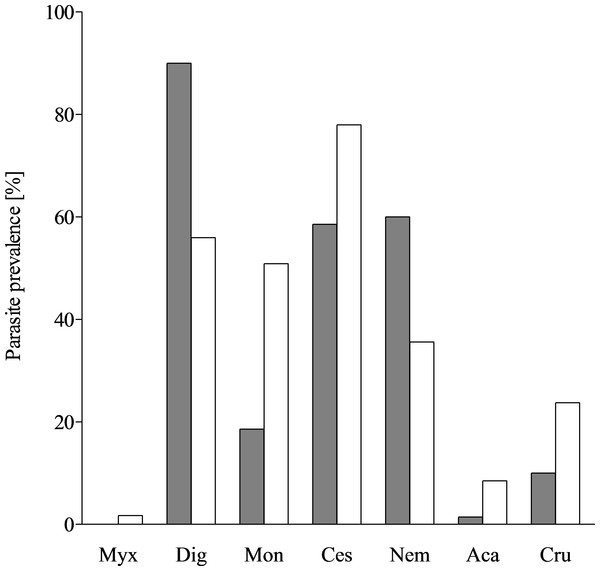
Due to the distribution of biodiversity throughout the water column, the parasite diversity was expected to be lower in the mesopelagial (N. tripes) than in the epipelagial (Trichiurus lepturus) (Angel, 1993). Both examined fish species have a distinct parasite fauna, the mesopelagic species N. tripes being infested by 13 parasite taxa from seven different groups, whereas T. lepturus was infested by at least nine parasite taxa from 6 different groups (Fig. 3). This finding contradicted the expectation, but as N. tripes is nyctoepipelagic, both species likely feed in the epipelagial. The differences in their parasitisation could be explained through the results of the stomach content analyses. With a piscivorous diet, N. tripes consumed mostly prey from a higher trophic level than T. lepturus, which had a large proportion of zooplankton in its diet. Thus, N. tripes is more likely to accumulate different parasites through its diet. The parasites occurring in both species were digenean larvae, early stages of cestode larvae, Anisakis typica and Hysterothylacium sp. The discovery of these parasites in both fishes examined is in accordance with the generalist lifestyle of the parasites and their records from various teleost hosts (e.g., Klimpel & Rückert, 2005; Carreras-Aubets et al., 2012; Kuhn et al., 2013). Anisakis typica is the dominant Anisakis species in tropical shelf regions (Mattiucci et al., 2002; Kuhn et al., 2013). The definitive hosts of A. typica are typically small coastal dwelling cetaceans which belong to the families Delphinidae, Phocoenidae and Pontoporiidae (Mattiucci et al., 2002). The fauna of small coastal Cetacea of the sampling area offers a diverse range of possible definitive hosts for A. typica, which is reflected in its relatively high prevalence in both T. lepturus and N. tripes (Dupuy & Maigret, 1976; Smeenk, Leopold & Addink, 1992; Hutterer, 1994; Robineau & Vely, 1997; Van Waerebeek et al., 1999; Felix & Van Waerebeek, 2005; Wenzel et al., 2009; Weir et al., 2011; Weir et al., 2014; Jung et al., 2016). A predator–prey relationship between dolphins from the genus Sotalia spp., which are a definitive host for A. typica, and T. lepturus was previously described (Melo et al., 2006; Carvalho et al., 2008). As a typical shelf inhabitant with a broad spectrum of intermediate hosts and pelagic life-cycle, infestation with A. typica was expected in the studied fishes.
Figure 3: Parasite prevalence in T. lepturus and N. tripes.
Prevalence of parasite taxa from T. lepturus (grey) and N. tripes (white). Myx, Myxosporea; Dig, Digenea; Mon, Monogenea; Ces, Cestoda; Nem, Nematoda; Aca, Acanthocephala; Cru, Crustacea.Conclusion
The discovery of mainly generalist parasites along with larval anisakid nematodes and cestode larvae reflects the typical parasite infestation of mesopredatory pelagic fishes. The abundance of nematode larvae is connected to the lower trophic level of the fishes, which consumed mainly planktonic Crustacea (T. lepturus) and small Teleostei (N. tripes). The low diversity and the presence of generalist parasites represents the species composition of an eastern boundary upwelling ecosystem: few dominant generalists with a high abundance, L. microstomum, digenean larvae and larval cestodes and a low overall species richness. An effect of upwelling seasonality on the parasite fauna could be examined in future studies.
Supplemental Information
Morphometric measures of Trichiurus lepturus and Nealotus tripes
ci, confidence interval; CW, carcass weight; GW, gonad weight; LW, liver weight; max, maximum; min, minimum; N.l., N. tripes; SD, standard deviation; SL, standard length; TL, total length; T.l., T. lepturus; TW, total weight.
Parasite fauna of Trichiurus lepturus according to online research
Reported parasites of T. lepturus, parasite taxa and prevalences (if available) from the literature with corresponding references. Asterisks indicate parasites discovered in the present study. BC, Brazil Current; CC, Canary Current; FC, Falkland Current; KC, Kuroshio Current; P, Prevalence; TWC, Taiwan Warm Current; ZMCC, Zhe-Min Coastal Current.
Raw data of morphometrics and diet of Trichiurus lepturus and Nealotus tripes specimens
ID, host identification code, Hol., catch number; SL, standard lenght; TL, total length; PL, preanal length; TW, total weight; CW, carcass weight; GW, gonad weight; LW, liver weight; SW, stomach weight.
Raw data of the parasitisation of Trichiurus lepturus and Nealotus tripes specimens
Ac, Acanthocephala, A. peg, Anisakis pegreffii, A. phy, Anisakis physeteris, A. sp., Anisakis sp., A. typ, Anisakis typica; Bo, Bomolochidae; C, Cestoda; Cal , Caligidae; Cr, Crustacea; D, Digenea; Dic, Diclidophoridae, Hem, Hemiuridae, H. sp., Hysterothylacium sp.; ID, host identification code; K. thy, Kudoa thyrsites; L. mic, Lecithochirium microstomum; L. sp., Lernaeenicus sp.; M, Monogenea; My, Myxozoa; N, Nematoda; N. sp., Nybelinia sp.; O. tra, Octoplectanocotyle travassosi; Tet , Tetraphyllidea; Try, Trypanorhyncha.