Communities of oil palm flower-visiting insects: investigating the covariation of Elaeidobius kamerunicus and other dominant species
- Published
- Accepted
- Received
- Academic Editor
- Ilaria Negri
- Subject Areas
- Agricultural Science, Biodiversity, Conservation Biology, Ecology, Entomology
- Keywords
- Scaptodrosophila, Central borneo, Oil palm flower, Sticky trap
- Copyright
- © 2019 Rizali et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Communities of oil palm flower-visiting insects: investigating the covariation of Elaeidobius kamerunicus and other dominant species. PeerJ 7:e7464 https://doi.org/10.7717/peerj.7464
Abstract
Insects visit flowers not only to forage for nectar or pollen but also to search for hosts or prey, and to look for suitable habitats for breeding sites. In oil palm flowers, it has been documented that not all flower-visiting insects are pollinators, but some insects are recognized as predators, parasitoids or saprophages, which may affect the abundance and persistence of the weevil pollinating oil palm, Elaeidobius kamerunicus. We studied the community of oil palm flower-visiting insects and investigated the covariation between the abundance E. kamerunicus and that of other dominant species. Ecological research was conducted in oil palm plantations with different tree ages in Central Borneo. Our results found that tree age and flower type of oil palm did not influence the abundance and species richness of flower-visiting insects, but significantly affected their species composition. There was a significant positive relationship between the abundance of E. kamerunicus and the fly Scaptodrosophila sp, indicating that these species covariate in oil palm flowers. These findings suggest that understanding the covariation between E. kamerunicus and Scaptodrosophila sp may help develop the conservation strategies for E. kamerunicus to support the sustainable production of oil palm.
Introduction
The presence of insects in oil palm flowers is related to their activity to look for nectar or pollen (Lajis, Hussein & Toia, 1985; Syed, 1979) or to search for prey (Hakim et al., 2017) as well as for suitable habitat for breeding sites (Corley & Tinker, 2003; Moore, 2001). The identity of the insects visiting oil palm flowers depends on the geographical region. In Africa, which is the origin area of oil palm plants, the most dominant flower visitors are Elaeidobius kamerunicus, E. plagiatus and E. subvittatus (Coleoptera: Curculionidae): these insects have an important role as a pollinators (Syed, 1979). In South America, the main pollinator of oil palm is Mystrops costaricensis (Nitidulidae), while in Asia it is Thrips hawaiiensis (Corley & Tinker, 2003). In Indonesia, since the introduction of E. kamerunicus in 1983, this weevil has become the most abundant oil palm flower-visiting insect, and its presence has been an important contribution to increasing fruit set of oil palm (Susanto, Purba & Prasetyo, 2007).
E. kamerunicus is well-adapted to wet tropical climates and found with high abundance in oil palm flowers in Indonesia (Prasetyo & Susanto, 2012). E. kamerunicus feeds and breeds in male inflorescences of oil palm (Corley & Tinker, 2003; Syed, 1979). Pollination occurs when E. kamerunicus, unintentionally carrying the pollen from male inflorescences on their elytra, visit female inflorescences. The weevil visits the receptive female inflorescences due to the attractive effect of estragole, a volatile compound released by female flowers that is similar to volatile compounds released by male flowers (Susanto, Purba & Prasetyo, 2007).
The production of oil palm, in terms of weight of bunches and the number of fruits set, has increased after the introduction of E. kamerunicus to Indonesia (Lubis, Sudarjat & Dono, 2017). A high oil palm fruit set (i.e., above 75%) requires a population of at least 20,000 E. kamerunicus individuals per hectare (Donough, Chew & Law, 1996). At present, oil palm cultivation is experiencing problems with decreasing fruit set (Prasetyo, Purba & Susanto, 2014; Teo, 2015). This is likely due to factors such as side effects of insecticide applications or increases in natural enemies of E. kamerunicus such as rats (Bessou et al., 2017), nematodes (Poinar et al., 2002), mites (Krantz & Poinar, 2004) or other predators (Hakim et al., 2017). For this reason, efforts are needed to increase the population of E. kamerunicus and to maintain the population above the minimum threshold needed to effectively pollinate the oil palm (Kahono et al., 2012). For instance, the population of E. kamerunicus can be increased in the field using the hatch and carry method (Prasetyo, Purba & Susanto, 2014). Further research is needed to better understand the drivers that affect the population of E. kamerunicus; it has, for instance, been shown that factors such as the tree age of palm oil (Rahardjo et al., 2018) as well as interactions with other flower-visiting insects (Hakim et al., 2017; Syed, 1979) affect the population of E. kamerunicus in the field.
Understanding the interaction between insect pollinators and other flower-visiting insects (anthophiles) is an importance aspect in ecosystem functioning and agricultural production (Kevan, 2008). As relatively primitive insect pollinators, Coleoptera and Diptera were documented on the fossil record as pollen vectors (Bernhardt, 2000; Kevan & Baker, 1983; Labandeira, 1998) and in recent times both insect groups can be found on the same plant, for instance in oil palm (Syed, 1979). Drosophilid flies (Diptera: Drosophilidae) are highly diverse as flower visitors and derive carbohydrate and utilize yeasts for their nutrition at flowers. Some species of curculionid beetles (Coleoptera: Curculionidae) were also reported to eat decomposed flowers (Moore, 2001; Syed, 1982). The presence of drosophilids and curculionids in the same flower may be associated with competition for resources, alternatively they may covary without any interaction.
In this research, we studied the community of oil palm flower-visiting insects in oil palm plantation in Central Borneo, Indonesia. We addressed the following questions: (i) which factors affect the communities of flower-visiting insects in oil palm plantations, and (ii) is there a relationship between the abundance of E. kamerunicus and that of other dominant species, while controlling for other factors? Information about covariation of flower-visiting insects is needed to understand ecosystem functioning and to develop a conservation strategy for pollinators of oil palm in Indonesia.
Materials & Methods
Research site and determination of sampling units
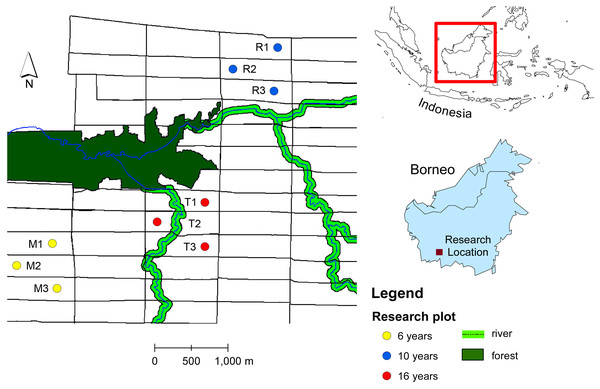
The ecological research was conducted in an oil palm plantation in Pangkalan Lada, Central Borneo, Indonesia. The tree age ranges from 4 years to 20 years. The oil palm with the same age were planted in a block with size 300 m × 1,000 m (30 ha) (Fig. 1). We chose productive oil palm plots with different tree ages: 6, 10 and 16 years-old. Within each age group, we selected three oil palm fields from different blocks. Within each oil palm field we selected a sampling plot. The sampling plot was a hundred oil palm trees (10 × 10 trees). The number of oil palm inflorescences varies in space and time due to environmental and plant genetic factors (Adam et al., 2011). To standardize the sampling unit, we sampled two anthesizing male inflorescences and two receptive female inflorescences in each plot, as this was the lowest number of oil palm flowers recorded from all plots across different tree ages (Table 1). The oil palm flower data were obtained by counting the number of anthesizing male and receptive female inflorescences in each plot before sampling. Every month, the number of male flowers ranges from 5–8 inflorescences per hundred trees, while female flowers range from 2–5 inflorescences per hundred trees.
Figure 1: Map of study sites in oil plantation in Central Borneo, Indonesia.
The letter and number refer to plot code listed in Table 1. Plots were selected in different tree age (6, 10 and 16 years) that located in different block of oil palm field with size of each block 300 m × 1,000 m (30 ha) and each block have the same tree age.| Tree age (year) | Plot code | No. of mature inflorescence (mean ± SD) | Average of tree height (m) (n = 25) | Light intensity (lux) (n = 15) | Vegetation diversity (n = 10) | Insect diversity | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Male | Female | Total | ||||||||||
| Male | Female | S | N | S | N | S | N | |||||
| 6 | M1 | 7.7 ± 1.5 | 4.7 ± 0.6 | 2.7 ± 0.3 | 293 ± 52 | 15 | 53 | 7,797 | 38 | 8,066 | 68 | 15,863 |
| M2 | 6.7 ± 1.5 | 3.7 ± 0.6 | 3.5 ± 0.3 | 310 ± 61 | 14 | 43 | 7,384 | 55 | 6,511 | 75 | 13,895 | |
| M3 | 7.0 ± 1.7 | 3.7 ± 1.2 | 4.0 ± 0.4 | 311 ± 45 | 14 | 70 | 11,513 | 69 | 15,264 | 106 | 26,777 | |
| 10 | R1 | 6.3 ± 0.6 | 4.0 ± 0.0 | 7.2 ± 0.4 | 255 ± 44 | 9 | 57 | 12,122 | 71 | 5,372 | 101 | 17,494 |
| R2 | 6.3 ± 1.5 | 4.0 ± 1.0 | 7.3 ± 0.6 | 259 ± 43 | 16 | 53 | 3,153 | 46 | 3,723 | 74 | 6,876 | |
| R3 | 5.7 ± 1.2 | 3.0 ± 0.0 | 6.8 ± 0.9 | 263 ± 46 | 9 | 49 | 6,746 | 55 | 10,115 | 74 | 16,861 | |
| 16 | T1 | 6.0 ± 1.7 | 2.0 ± 0.0 | 9.1 ± 0.5 | 214 ± 25 | 19 | 46 | 14,802 | 63 | 11,580 | 79 | 26,382 |
| T2 | 7.3 ± 0.6 | 2.3 ± 0.6 | 9.6 ± 0.6 | 227 ± 31 | 20 | 47 | 14,855 | 48 | 22,896 | 69 | 37,751 | |
| T3 | 7.3 ± 0.6 | 2.0 ± 0.0 | 10.1 ± 0.7 | 236 ± 34 | 12 | 50 | 10,995 | 39 | 8,680 | 65 | 19,675 | |
| Total | 199 | 89,367 | 198 | 92,207 | 275 | 181,574 | ||||||
We also measured the plot characteristics including tree height, light intensity and understorey vegetation diversity on each plot. Light intensity was measured using a lux meter that was set up close to male and female inflorescences. While the observation of understorey vegetation was done in 10 randomly placed 1 × 1 m quadrats. In all blocks, the management of understorey vegetation was managed by grazing with cows, without herbicide application. The diversity of understorey vegetation at each point was noted and the specimen samples were taken or photographed to be identified in the laboratory. Identification of vegetation specimens was conducted using the reference of Xu & Zhou (2017).
Sampling and identification of oil palm flower-visiting insects
The sampling of oil palm flower-visiting insects was done by installing a sticky trap in two male and two female inflorescences in each plot. The sticky traps were made from transparent plastic with size 15 cm ×10 cm and smeared with an adhesive material (rat glue). Five traps were mounted circularly covering all parts of an inflorescence and were installed during the day (07.00 am–16.00 pm) and the night (16.00 pm–07.00 am) to collect flower visitors both of diurnal and nocturnal insects. Trapped insects then were preserved using 70% alcohol for further sorting and identification in the laboratory. In each plot, insect sampling was conducted every month in different inflorescences, during three months from March to May 2016.
Specimens of flower-visiting insects were initially sorted to order and family level using the identification books such as Borror, Triplehorn & Johnson (1996), Goulet & Huber (1993) and McAlpine (1987). Afterwards, each order or family of insects was then identified to morphospecies level based on the differences of morphological characters and if possible until genera level especially for ants (using Bolton, 1994) and flies (using Bock, 1976).
Data analysis
The difference of dominant insect abundance between male and female inflorescences was tested using analysis of variance (ANOVA). Effect of environmental factors on the richness and abundance of flower-visiting insects was analyzed by fitting a generalized linear model (GLM) without interactions (Zuur et al., 2009) and using a quasiPoisson distribution to account for overdispersion. Explanatory variables included tree age of oil palm, flower type (male/female), and vegetation diversity. We excluded tree height (Pearson’s r = 0.962, P < 0.001) and light intensity (Pearson’s r = −0.955, P < 0.001) due to strong correlation with tree age of oil palm.
The effect of environmental factors on species composition of flower-visiting insects was analyzed by canonical correspondence analysis (CCA) and continued using forward selection with 1,000 permutations. In addition, pairwise test from analysis of similarity (ANOSIM) with the Bray-Curtis index was also used to compare insect species composition between different tree ages of oil palm (Legendre & Legendre, 1998).
Covariation between E. kamerunicus and other dominant insect species was analyzed using GLM with the abundance of dominant species (Scaptodrosophila sp, Pheidole sp and Gelechiidae sp), tree age, flower type of oil palm, and vegetation diversity as explanatory variables.
All analyzes were performed using R statistical software (R Core Team, 2018) and utilizing the vegan package for CCA and ANOSIM (Oksanen et al., 2015).
Results
Diversity and species composition of oil palm flower-visiting insects
The diversity of oil palm flower-visiting insects recorded across all plots was 275 species from 10 orders and 181,574 individuals (Tables 1 and 2). The Coleoptera were most abundant and dominated by E. kamerunicus (Fig. 2A). Other dominant insects were Diptera, dominated by Scaptodrosophila sp, Hymenoptera which were dominated by ants (Pheidole sp) and Lepidoptera which were dominated by a moth species (Gelechiidae sp) (Table 2, Figs. 2B–2D). The abundance of Coleoptera (F1,52 = 0.342, P = 0.561) and Lepidoptera (F1,52 = 0.012, P = 0.914) were not different between male and female inflorescences. In contrast, the abundance of Diptera was significantly higher in male inflorescences (F1,52 = 35.490, P < 0.001), while Hymenoptera were more abundant in female inflorescences (F1,52 = 4.057, P = 0.049).
| No | Order | Male | Female | Total | Dominant species (% of N total) | |||
|---|---|---|---|---|---|---|---|---|
| S | N | S | N | S | N | |||
| 1. | Blattodea | 1 | 7 | 1 | 3 | 1 | 10 | |
| 2. | Coleoptera | 20 | 75,320 | 16 | 84,098 | 28 | 159,418 | Elaeidobius kamerunicus (99.9%) |
| 3. | Dermaptera | 2 | 5 | 4 | 8 | 5 | 13 | |
| 4. | Diptera | 97 | 11,603 | 81 | 4,282 | 121 | 15,885 | Scaptodrosophila sp (89.1%) |
| 5. | Hemiptera | 6 | 18 | 7 | 9 | 8 | 27 | |
| 6. | Homoptera | 10 | 11 | 8 | 9 | 13 | 20 | |
| 7. | Hymenoptera | 47 | 565 | 62 | 1,989 | 78 | 2,554 | Pheidole sp (55.9%) |
| 8. | Lepidoptera | 6 | 1,790 | 8 | 1,755 | 10 | 3,545 | Gelechiidae sp (94.4%) |
| 9. | Mantodea | 1 | 1 | 1 | 1 | 1 | 2 | |
| 10. | Orthoptera | 9 | 47 | 10 | 53 | 10 | 100 | |
Figure 2: The most dominant species of flower-visiting insects in oil palm plantation in Central Borneo, Indonesia.
(A) Elaeidobius kamerunicus, (B) Gelechiidae sp, (C) Scaptodrosophila sp, and (D) Pheidole sp.The results of GLM showed that tree age, flower type of oil palm and vegetation diversity did not influence the species richness and abundance of flower-visiting insects (Table 3). In addition, the CCA revealed that the species composition of flower-visiting insects was significantly affected by flower type and tree age of oil palm (Table 4). The ANOSIM results also proved that the composition of flower-visiting insects differed between flower type (R = 0.039, P = 0.046) and tree age (R = 0.113, P = 0.001). Species composition of flower-visiting insects was significantly different between palms 6 and 16 years old, as well as between palms 10 and 16 years old, but not between palms 6 and 10 years-old (Table 5).
| Variable | Species richness | Abundance | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | |
| (Intercept) | 3.470 | 0.151 | <0.001 | 6.901 | 0.324 | <0.001 |
| Tree age | −0.011 | 0.008 | 0.186 | 0.034 | 0.020 | 0.089 |
| Vegetation diversity | −0.006 | 0.009 | 0.506 | 0.033 | 0.021 | 0.129 |
| Flower type (male) | −0.027 | 0.063 | 0.669 | −0.031 | 0.141 | 0.825 |
| Plot (2) | 0.154 | 0.079 | 0.057 | 0.458 | 0.186 | 0.018 |
| Plot (3) | 0.191 | 0.078 | 0.019 | 0.486 | 0.185 | 0.012 |
| Month (2) | −0.196 | 0.077 | 0.014 | −0.187 | 0.185 | 0.318 |
| Month (3) | −0.141 | 0.076 | 0.068 | 0.267 | 0.166 | 0.114 |
| Variable | DF | AIC | F | P-value |
|---|---|---|---|---|
| Flower type | 1 | 393.52 | 3.926 | 0.005 |
| Tree age | 1 | 395.46 | 1.950 | 0.020 |
| Vegetation diversity | 1 | 396.22 | 1.197 | 0.135 |
| Tree age | R | P-value |
|---|---|---|
| 6 years vs. 10 years | 0.052 | 0.074 |
| 6 years vs. 16 years | 0.076 | 0.037 |
| 10 years vs. 16 years | 0.206 | 0.001 |
Covariation in abundance of E. kamerunicus and other dominant species
We focused on the covariation of E. kamerunicus with the other dominant species in the flower-visiting community: Scaptodrosophila sp, Pheidole sp and Gelechiidae sp (Table 2). The results of GLM showed that the abundance of E. kamerunicus was positively affected by abundance of Scaptodrosophila sp (P = 0.001), vegetation diversity (P = 0.007) and female flower type (P = 0.008) (Table 6). The same pattern for abundance of Scaptodrosophila sp was also positively affected by abundance of E. kamerunicus (P = 0.001), vegetation diversity (P = 0.009) and flower type (P < 0.001) (Table 6).
| Variable | E. kamerunicus | Scaptodrosophila sp | ||||
|---|---|---|---|---|---|---|
| Estimate | SE | P | Estimate | SE | P | |
| (Intercept) | 6.144 | 0.386 | <0.001 | 4.910 | 0.382 | <0.001 |
| Scaptodrosophila sp | 0.002 | 0.000 | 0.001 | |||
| E. kamerunichus | 0.000 | 0.000 | 0.001 | |||
| Pheidole sp | 0.000 | 0.001 | 0.892 | 0.001 | 0.001 | 0.624 |
| Gelechiidae sp | 0.002 | 0.002 | 0.202 | 0.000 | 0.002 | 0.997 |
| Tree age | 0.006 | 0.022 | 0.798 | 0.028 | 0.022 | 0.196 |
| Vegetation diversity | 0.064 | 0.022 | 0.007 | −0.056 | 0.020 | 0.009 |
| Flower type (male) | −0.537 | 0.193 | 0.008 | 1.158 | 0.164 | 0.000 |
| Plot (2) | 0.366 | 0.208 | 0.086 | 0.172 | 0.191 | 0.374 |
| Plot (3) | 0.655 | 0.194 | 0.002 | −0.251 | 0.191 | 0.196 |
| Month (2) | −0.148 | 0.189 | 0.438 | −0.030 | 0.172 | 0.864 |
| Month (3) | 0.360 | 0.164 | 0.034 | −0.151 | 0.172 | 0.384 |
Discussion
The most dominant oil palm flower-visiting insect in oil palm plantations in Central Borneo is E. kamerunicus. An introduced species, this weevil has adapted well to Indonesian oil palm plantations, yet their populations have been shown to be prone to decline (Prasetyo, Purba & Susanto, 2014). The second dominant species was Scaptodrosophila sp, a member of the drosophilid flies that is widespread in tropical Asia and known to feeding and breeding sites in fruit, flowers and leaves (Bock & Parsons, 1978). In oil palm plantations, Scaptodrosophila sp was found in high abundance in male inflorescences. It indicated that male inflorescence of oil palm contains food sources and suitable sites for breeding of Scaptodrosophila sp. Barker (2005) showed that species of Scaptodrosophila are restricted to flowers of certain plant species for feeding and breeding.
The ant species Pheidole sp was also found dominant in oil palm flowers. Kahono et al. (2012) reported that ants actively visit the flowers of oil palm both on receptive female inflorescence and anthesizing male inflorescence. The role of ants in oil palm flowers may include foraging for nectar or for prey, but this has to our knowledge never been investigated further. Nectar is an attractant for flower visiting insects including pollinators, herbivores, predators or parasitoids (Strauss & Whittall, 2006). In addition, a moth morphospecies (Gelechiidae sp) was also found dominant in oil palm flowers. As nocturnal insects, moths visit oil palm flowers during the night to find flower nectar and their feeding activity also have a contribution to pollination (Moore, 2001). However, E. kamerunicus is the most effective pollinator of oil palm due to its ability to carry many pollen grains compared with other Elaeidobius species (Kouakou et al., 2014) and other potential pollinators such as the moths Pyroderces sp. (Momphidae) and Thrips hawaiiensis (Corley & Tinker, 2003; Moore, 2001). Male weevils carry more pollen than female weevils because they have a larger body size and more setae (Moore, 2001). Surprisingly, T. hawaiiensis, as a former potential pollinator in Asia (Corley & Tinker, 2003) was not recorded in this research.
In this study, we found that tree age of oil palm did not affect the species richness and abundance of flower-visiting insects. However, increasing tree age affected the species composition of flower-visiting insects. As a consequence of increasing tree age, the architecture of oil palm plants such as tree height and a canopy is also changing. This may increase the availability of nest sites and microhabitats for insects, thus shaping the diversity as well as species composition of insects in oil palm plantation. Research by Sahari (2012) revealed that insects, and especially parasitoid wasps, were more diverse in the open canopy with more sunlight. Open canopy also facilitates the diversity of understorey vegetation especially flowering plants that provide alternative habitat and food source for pollinator insects (Klein, Steffan-Dewenter & Tscharntke, 2003) as well as natural enemies (Perovic et al., 2010). In cacao agroforestry system, increasing age of cacao tree changed the architecture of cacao tree as well as shade trees and affected the species composition of ants (Rizali et al., 2013).
The difference of flower types also affected the species composition of oil palm flower-visiting insects. Male and female inflorescences have different structural morphologies in which male flower have pollen and nectar and different volatile compounds compared to the female flower; therefore, it affects preference for the visiting insects (Moore, 2001; Syed, 1979). However, the receptive female flower of oil palm produces estragole, a volatile compound that is also produced by the male flower, and that attracts E. kamerunicus to visit despite absence of food or nesting site in female flowers (Susanto, Purba & Prasetyo, 2007).
The analysis of the relationship between E. kamerunicus and other dominant species, revealed that the abundance of E. kamerunicus is positively correlated to the abundance of Scaptodrosophila sp, while controlling for environmental variables. Scaptodrosophila sp, like E. kamerunicus, is arguably utilizing male flower of oil palm for feeding and breeding sites, while other dominant insects, ants and moths were merely looking for nectar. The difference between E. kamerunicus and Scaptodrosophila sp was that the abundance of Scaptodrosophila sp was higher in male than in female inflorescences, with E. kamerunicus showing no such difference. The covariation between E. kamerunicus and Scaptodrosophila sp in oil palm flowers was presumably related to the similar behaviour of both species as fungus-eating insects (mycophagous). Coexistence between fungus-eating insects is well known from other systems (Kadowaki, 2010). In Africa, E. kamerunicus may coexist with other fungus weevils such as Nitidulidae and Mycetophagidae (Syed, 1979) which have an important role in decomposition processes. Biological studies showed that E. kamerunicus do not eat pollen, the adults feed only the inside part of a male flower of oil palm and larvae develop on decomposed flowers (Moore, 2001; Syed, 1982). Feeding activity of the weevils may facilitate the growth of fungi and bacteria for the decomposition process of waste food material. The presence of fungi and bacteria may attract Scaptodrosophila sp to visit the oil palm flowers for feeding and breeding (Jacome et al., 1995).
Bacteria and fungi have an important role for drosophilid flies as food sources and increasing their fitness. Therefore, drosophilids transfer both bacteria and fungi during mating. Bacteria are the most important microbes for decomposition, while fungi (yeasts) play a role in fermentation (Markow & O’Grady, 2008). Drosophilids are attracted to visit and oviposit by ethanol (Hoffmann & Parsons, 1984) which may be produced by yeast during the decomposition process of waste material that has been utilized by E. kamerunicus. In addition, drosophilids also deposit bacteria and fungi in breeding sites during eggs laying to increase the food resource for larvae.
Conclusions
This study found that the abundance of E. kamerunicus is not only positively related to the vegetation diversity within oil palm plantation, but also to the abundance of Scaptodrosophila sp. Although the mechanism is uncertain yet, it is a possibility that E. kamerunicus has mutualistic interaction with Scaptodrosophila sp. Further study is needed to investigate the interaction mechanism between E. kamerunicus and Scaptodrosophila sp as well as their symbiont microbes. We believe that understanding those interactions will provide significant benefit for conservation and management strategy of E. kamerunicus in oil palm plantation (Li et al., 2019), beside understanding the biology of E. kamerunicus (Tuo, Koua & Hala, 2011), releasing E. kamerunicus to increase pollination (Prasetyo, Purba & Susanto, 2014) as well as controlling predators and other natural enemies of E. kamerunicus (Hakim et al., 2017).
Supplemental Information
Diversity of flower-visiting insects in oil palm plantation in Central Borneo, Indonesia that collected from both male and female inflorescence in different tree age of oil palm (6, 10, 16 years) during 3 months of sampling
Species accumulation curves of flower-visiting insects between
(a) all plots and different tree ages, (b) plots with tree age 6 years, (c) plots with tree age 10 years and (d) plots with tree age 16 years.