Zero-valent iron nanoparticles for environmental Hg (II) removal: a review
- Published
- Accepted
- Received
- Academic Editor
- Sreeprasad Sreenivasan
- Subject Areas
- Materials Science (other), Nano and Microstructured Materials
- Keywords
- Zero-valent iron nanoparticle, Adsorption, Mercury removal
- Copyright
- © 2023 Dan-Iya et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ Materials Science) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Zero-valent iron nanoparticles for environmental Hg (II) removal: a review. PeerJ Materials Science 5:e29 https://doi.org/10.7717/peerj-matsci.29
Abstract
Mercury is a natural, long-lasting, and bio-accumulative contaminant found in both soil and water. Mercury is toxic and its organic derivative, methylmercury (MeHg), could be lethal. The increasing level of mercury in the environment is a threat, as it can easily enter the food chain upon exposure. Zero-valent iron nanoparticle (nZVI), an environmentally friendly nanomaterial, is envisaged as an ideal candidate for the remediation of metal pollutions in soil and water bodies. Due to low toxicity and decent activity, nZVI and its corrosion products have shown huge potential for the removal of heavy metals from soil and water. It has been widely applied for the removal of heavy metals including mercury and other organic and inorganic contaminants. In this review, the current preparation methodology, characterization techniques, reductive mechanism for heavy metal removal with focus on mercury is reviewed. This review discusses the use of nZVI for the removal of mercury and demonstrates that nZVI possesses high reactivities for mercury removal and have great application prospects in environmental remediation. Some recommendations are proposed and conclusions drawn for future research.
Introduction
The increased industrial activities since the past century have led to the contamination of soil and the aquatic environment with toxic metals and organics that pose significant risks and hazards to the ecosystem and human health. These pollutants get into the environment via different natural as well as anthropogenic sources including weathering of rocks, burning of coal, improper waste disposal, industrial discharge, etc. (Mondal et al., 2022; Ozsoy, 2010; Sun et al., 2017).
The rapid industrialization and urbanization have elevated the demand for the industrial use of heavy metals in various fields such as automobiles, coating materials, explosives, aeronautics, photographic films, storage batteries pigments, and steel industries (Jain et al., 2021; Gheju, 2011; Hu, Yang & Wang, 2012; Liu et al., 2013; Ren et al., 2013). Besides their propitious role, heavy metals pose an environmental risk and potential hazards as they also account for the polluting of the environment and as such draw considerable attention to human well-being and safety, not to mention environmental stability (Shao, Chen & Wang, 2012; Zhang et al., 2014). Ion exchange, reverse osmosis, adsorption with adsorption being the most important method (Shuhan et al., 2023; Weiyu et al., 2022), and chemical precipitation (Almomani et al., 2020; Khurshid, Mustafa & Isa, 2022) are among the methods used in the removal of heavy metals and organic pollutants. However, the series of processes involved in removing heavy metals are harder compared to organic pollutants (Liu et al., 2013). Such heavy metals include hexavalent chromium (Cr(VI)), lead (II) ion (Pb(II)), copper (II) ion (Cu(II)), zinc (II) ion (Zn(II)), mercury (II) cation (Hg(II)), and nickel (II) ion (Ni(II)) (Table 1). These heavy metals usually accumulate in living organisms and pose a potential threat of toxicity since most of these heavy metals are carcinogenic (Chen et al., 2014; Liu et al., 2013; Zhang et al., 2013; Zhao et al., 2013). Lead poisoning, for example, can cause severe injuries through multiple toxicity and progressive accumulation to essential processes and activities of the cell, brain functions, liver or kidney (Arshadi et al., 2014; Hu et al., 2015; Karabelli et al., 2008; Zhang et al., 2014). The process of efficiently removing detrimental toxicity anchored by heavy metals as part of an attempt to maintain environmental strength and community protection is exceptionally important and a demanding area of research towards ecological remediation.
| Metal | Metal ions | Natural sources | Anthropogenic source | Uses | Health effects | Ref. |
|---|---|---|---|---|---|---|
| Chromium, (Cr) | Cr6+ | Ultramafic rocks | Mining of chromite (FeCr2O4) as the main source | Metallurgy accounting for 67%, refractories 18% and chemical usage of 15%. | Effects on nasal mucosa and skin. Lung cancer and other respiratory diseases | Saha, Nandi & Saha (2011) |
| Lead (Pb) | Pb2+ | Galena mineral | Pigments, coal, battery plants, pipelines, and gasoline | Alloys and Batteries | Neurotoxic, brain damage, anemia, IQ diminishing, loss of appetite and malaise | Hannahs (2011) |
| Copper (Cu) | Cu2+ | Carbonates, Oxides and Sulfides | Industrial and domestic waste, alloys, waste from mining, pesticides | Electrical conductor | Neurotoxicity, reproductive toxicity, dizziness, diarrhea, anemia | Hannahs (2011) |
| Zinc (Zn) | Zn2+ | Oxides, Sulfides, Silicates | Metal alloys, pigments, electroplating, industrial waste, pipelines | Pigments, Fertilizers, and plastics | Vomiting, diarrhea, dizziness, headache, anemia abdominal pain, dehydration, and stomach irritation | Hannahs (2011) |
| Mercury (Hg) | Hg2+ | Petroleum, Coal & Cinnabar, Forest fires and Volcanoes | Hydroelectric discharge, mining, paper industries, pulp, medical waste, and power plant emissions. | Recovery of Au and Ag, pharmaceuticals, agricultural chemicals, paints, measuring instruments, and electrical components | Damage the cardiovascular system, kidneys, liver, and bones | (Caner, Sarl & Tüzen, 2015; Steinnes, 2013) |
| Nikel (Ni) | Ni2+ | Soil | Battery plants, alloys, industrial waste vegetables production | Batteries, electronics, catalysts | Cardiovascular diseases, bronchitis, lung fibrosis, allergies of the skin, lung fibrosis, lung cancer | Hannahs (2011) |
Mercury is a naturally occurring heavy metal and has a long-lasting effect on living organisms (Amiri, Abedi-Koupai & Eslamian, 2017). The mercury level in the atmosphere has risen significantly due to its use in agriculture and various industries (e.g., chloro-alkali productions, medicinal as well as cosmetic formulations, electrical devices, pulp and paper factories, etc.) along with household goods (e.g., thermometers, batteries, medical drugs, etc.) (Ozsoy, 2010). Normal processes like volcanic eruptions and weathering, as well as anthropogenic practices like fuel burning, logging, non-ferrous metal smelting, municipal wastes, chlor-alkali industries, and the incinerations of waste and sludge can all result to a rising amount of mercury in water (Amiri, Abedi-Koupai & Eslamian, 2017). In the environment, soils and sediments are usually its final sink where it gets easily methylated to methyl mercury, a predominant form. Methyl mercury can readily be absorbed by biota and thus can reach and accumulate in humans via the food chain.
Mercury is one of USEPA’s priority toxic compound and considered to be highly poisonous at concentrations of more than 6 parts per billion (ppb). The permissible Hg (II) ions limitation is 0.001 mg L−1 for drinking water and 0.005 mg L−1 for wastewater. The European Communities Council of 1976 had ascertained that a higher concentration of mercury and lead can result to a broad array of detrimental challenges for adults and children as regards health (Amiri, Abedi-Koupai & Eslamian, 2017). The blood–brain barrier can quickly allow its penetration, leading to damage of the foetal brain (Zabihi, Haghighi Asl & Ahmadpour, 2010). Hg has high affinity to protein binding, and it predominantly affects the renal and nervous systems (Byrne & Mazyck, 2009). High levels of mercury (II) affect pulmonary and kidney function, as well as inducing chest pain and dyspnea (Inbaraj & Sulochana, 2006; Rao et al., 2009; Yavuz et al., 2006). The Minamata incident in Japan, caused by consuming contaminated seafood in the 1950s, is one dramatic example of mercury poisoning (Diagboya, Olu-Owolabi & Adebowale, 2015). The removal of mercury from wastewater to at least a low level is extremely necessary. Therefore, polluted water must be treated before discharged into natural water bodies to reduce the harmful effects of Hg (Ozsoy, 2010).
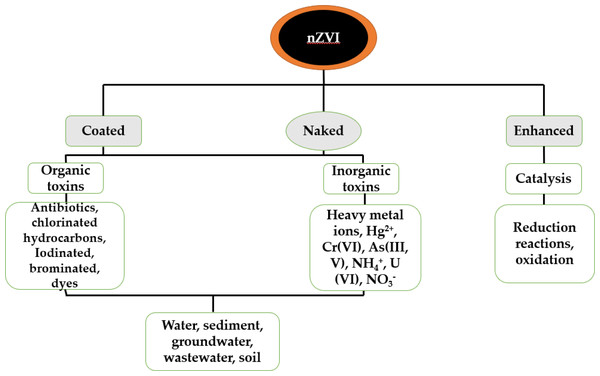
A number of techniques based on nanotechnology are in use for water treatments such as nanofiltration (carbon nanotubes and alumina fibers for nanofiltration) adsorption, ultrafiltration, membrane separation, catalytic reduction (zero valent metals) electro-dialysis, etc. The use of nanoreagents such as zero-valent iron (ZVI) and nano-catalysts is a new and promising trend in environmental remediation due to their high reaction rate coupled with a sufficient superficial area. For instance, ZVI was used in the decolorization of Azo dye (Khan et al., 2017), zero-valent iron nanoparticles (nZVI) is generally preferred in contrast to some many adsorbents due to its high reactivity, stronger magnetic property (Yang et al., 2019), larger and stabilized surface area (Bardos et al., 2016). These properties of nZVI potentially render it advantageous over the usual granular/microscale particles. Several reports are available on zero valent iron nanoparticles for the removal or elimination of contaminants with either organic and inorganic origin from water or soil (Pasinszki & Krebsz, 2020). Polychlorinated biphenyls, chlorinated hydrocarbons, chlorinated ethane, aromatic hydrocarbons, and heavy metals are examples of such contaminants (Bardos et al., 2016). Figure 1 illustrates various aspects of zero-valent iron nanoparticle’s relevance in nano remediation and water purification. The rationale behind the review literature is to ascertain the level of mercury toxicity and to describe ways to deal with it.
Figure 1: Various application fields for nZVI in nanoremediation as well as treatment of water (Pasinszki & Krebsz, 2020).
In this review, we have included discussion on Hg, its occurrence, natural sources, environmental fate, and possible health hazards related to the harmful effects of mercury on human health and the environment. The method for removal of Hg using zero-valent iron nanoparticle coupled with the associated benefits is explained. The synthesis protocol and mechanism of nZVI action for the removal of mercury is presented. The possible toxicity posed by nZVI on aquatic and terrestrial life along with ways to reduce and find a trade-off between toxicity and activity is also discussed. Future projections and shortcomings in the removal of mercury using nZVI are explained before a conclusion is drawn. The targeted audience of this write-up is not only researchers in the material science, environmental remediation or ecotoxicological field but also the public.
Search methodology
Various journals and journal articles were consulted and referenced via three search engines; Google, Yahoo, and Microsoft Bing and through different research websites including ResearchGate, Google Scholar, Science Direct, Wiley Online Library, Nature, Academia, and various other research website outlets to understand the toxic effect mercury causes and its adsorption remediation by zero valent iron nanoparticles.
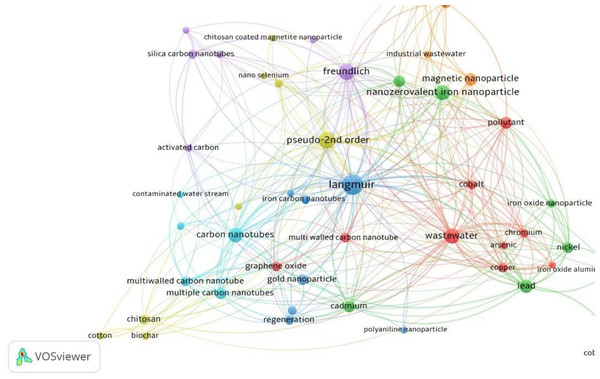
To better reflect the usage of nanoparticles in mercury sorption, a Scopus keyword search focused on the topics of “mercury”, “nanoparticle”, “Langmuir”, and “sorption”, yielding 49 journal articles. The VOSViewer software is used to create the keyword co-occurrence of the 49 documents, which is displayed in Fig. 2. The study’s goal is to conduct a complete bibliometric assessment of the research landscape of mercury sorption based on nanoparticles. Seven clusters were discovered, and the term nanozerovalent iron nanoparticle occurs 32 times. The isotherms Langmuir, Freundlich, Redlich Peterson and BET occur 28,11,1 and 1 time, respectively, suggesting that the Langmuir is the dominant isotherms in governing the sorption of mercury to nanoparticles. Of the kinetics model utilized, the pseudo-2nd order occurs 11 times whilst the pseudo-1st order occurs two times. The pseudo-2nd order kinetics is more predominantly reported in the literature (Allen et al., 2005; Febrianto et al., 2009; Namasivayam & Sangeetha, 2008) partly due to its mathematical model advantage compared to pseudo-1st order (Plazinski, Rudzinski & Plazinska, 2009).
Figure 2: Bibliometric map generated by VOSviewer based on Scopus Collection.
The keywords were “mercury”, “nanoparticle”, “Langmuir”, and “sorption”, yielding 49 journal articles.Synthesis of zero-valent iron nanoparticles
ZVI particles have shown great potential for cleaning chlorinated organic compounds, toxic metal cations as well as organic and inorganic contaminants from water and soil (Liang et al., 2021). ZVI is a moderate-reducing agent that can react with dissolved oxygen to produce reactive oxidants capable of oxidative water treatment (Zhang et al., 2019). ZVI is a core–shell nanoparticle as previous findings indicated that there is a special core–shell arrangement in ZVI (Liang et al., 2021). Because of their potential uses, core–shell nanomaterials and nanostructures have been a significant study field in recent decades (Kumar, Kumar & Paik, 2013). The core–shell nanomaterials and nanoparticles can have varying core as well as shell thicknesses and surface morphologies. (Kumar, Kumar & Paik, 2013). Core–shell nanostructures are biphasic nanomaterials with an internal core structure and an outside shell consisting of several constituents. These nanoparticles have caught the attention of researchers because they can display unique features due to the mix of core as well as shell structure, shape, and design (Nomoev et al., 2015). When there is functional groups or molecules modification or coated with a thin layer of other materials on the surface of the nanomaterial, they usually exhibit enhanced characteristics in comparison with the nonfunctionalized or uncoated materials (Kumar, Kumar & Paik, 2013). They have also been engineered such that the shell component can increase the reactivity, thermal stability, and or oxidative stability of the core nanomaterial, or to employ a cheap core material to transport a thin, more expensive shell material (Nomoev et al., 2015). As a result, they have a wide range of applications in domains such as biomedicine, electrical and semiconducting materials, and catalysts. (Nomoev et al., 2015).
The core in ZVI nanoparticle consists of Fe0, which provides electrons and excellent reduction capability, while the shell is iron oxides that provide potential for adsorption (Liang et al., 2021). Granular (>50 µm), or micro sized ZVI, has been applied as a reactive material for the removal of different pollutants in permeable reactive barriers (PRBs) (Üzüm et al., 2009). It has also been used for the removal of other contaminants such as nitrate, azo-dyes (Calderon & Fullana, 2015) etc. Therefore, it is considered a promising candidate for a rapidly emerging prominent technology with considerable potential benefits (Tratnyek & Johnson, 2006). The chemical, catalytic, electronic, magnetic, mechanical, and optical properties tend to change at the nano level owing to their small size and large surface area (Jortner & Rao, 2002), such that nanomaterials are expected to be more reactive than their granular and micron-sized counterparts. Nano-sized ZVI can enhance the remediation efficiencies of the contaminants quite remarkably (Calderon & Fullana, 2015).
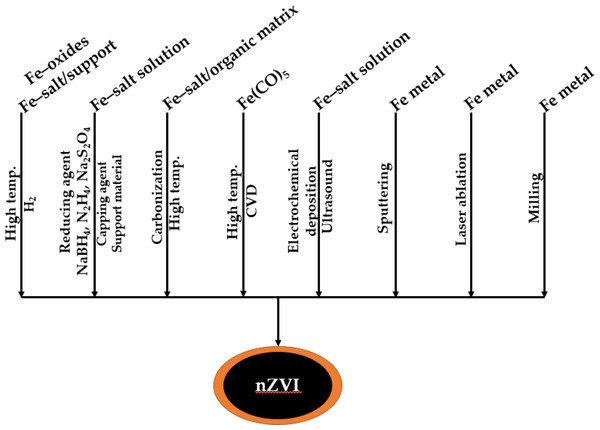
Numerous strategies employed to produce nZVI particles have been reported in the literature, wherein the synthetic approach can be broadly classified into two categories, i.e., the top-down and bottom-up approach described in Fig. 3 (Alazaiza et al., 2022). In a top-down approach, chemical and/or mechanical methods like etching and grinding (milling) are used to convert bulk material into smaller pieces using mechanical, chemical or other forms of energy. However, in a bottom-up approach, the material is synthesized from scratch at the atomic level by joining atom to atom or at the molecular level by combining molecule with molecule, thereby making a large composite of nanoparticulate structures, which is usually achieved by self-assembling, chemical synthesis, positional assembling, etc. (Ponder, Darab & Mallouk, 2000). Both approaches can be used in either gas, liquid, supercritical fluids, solid states, or in a vacuum. The structure of the nZVI particles produced by different procedures varies, giving rise to varied reactivity and, as a result, a potential variance in remediation efficacy.
Figure 3: Bottom-up & top-down methods of zero-valent iron nanoparticle synthetic production (Pasinszki & Krebsz, 2020).
Top-down method
The top-down nanoscale zero-valent iron approach includes mechanical grinding (ball milling), chemical etching, laser ablation, sputtering, electro-deposition, etc. The milling process is the most widely used top-down procedure. It begins with iron fillings of micrometer—millimeter-sized (e.g., Fe powder, sponge Fe, cast Fe as well as carbonyl Fe, cast Fe as well as Fe fine particles) that are grinded (utilizing stirred balled-milling, vibrating-mills, ball-mills and stirred-ball-millings) into small nanoscale flakes. This approach is also used in industries to manufacture nZVI on a wide scale. The most prominent benefit of this approach is scalability, i.e., ramped up production as per demand. Additionally, the treatment does not necessitate the use of costly and hazardous chemicals. The dimension as well as form of final nanoparticles are determined by the milling process that possesses significant influence on the reactivity of particulate material. Iron particles, milled in an inert environment, are highly reactive and pyrophoric, igniting violently upon contact with oxygen. To reduce the risk of combustion and reactivity of nanoparticles, a capping layer is normally formed during the milling process in a grinding medium. Stabilizing oxide shell occurs in nanoparticles while the media absorbs water, but the side product hydrogen produces a combustion hazard. Grinding machines and a variety of grinding media and additives (the time of milling, source of iron) were investigated to address stability and safety issues (Pasinszki & Krebsz, 2020). Recently, Golder Associates Inc. has become the leading big-size market area manufacturer of nZVI. The company generates this nanoparticle in huge amounts using the top-down method along with the mechanical-grinding of larger iron molecules in universal ball milling arrangements. Although the manufacturing process appears easy, nanoparticles generated demonstrate an exceptionally elevated surface-energy, hence susceptible to accumulation (Hwang, Kim & Shin, 2011).
Laser ablation is another prominent and green technique used to produce nZVI. The nanoparticles are fabricated and dispersed in liquid by ablation of metal plates using a high-power laser. When the laser beam reacts with the metal target, a plasma plume of photoionized metal ions is formed. Nanoparticle nucleation happens during the cooling of the plasma plume. Nuclei development and coalescence are the fundamental processes in the formation of metal nanoparticles by laser ablation. Energy, laser repetition rate, wavelength, ablation time, and aqueous solution absorption are all important factors in metal nanoparticle synthesis (Reza Sadrolhosseini et al., 2019). The chemical etching, electrodeposition and sputtering methods are less commonly used for the synthesis of ZVI nanoparticles.
Bottom–up method
The bottom-up approach is a technique for creating material from atomic or molecular constituents through chemical interactions. It involves a self-assembly approach of the miniaturized material components into nanostructures using physical forces operating at the nanoscale. Physical forces at the nanoscale are utilised to join basic components into forming bigger and more stable structures (Wolfgang, 2004). Depending on the chemicals used for the synthesis of nZVI, the bottom-up approach can be classified as the chemical route (sodium borohydride and hydrazine), semi-green route (sodium dithionite), and green route (plant extract) for nZVI synthesis.
Chemical route (sodium borohydride and hydrazine)
For the development of highly reactive nZVI, a chemical synthesis using an iron salt and sodium borohydride is a widely used process that needs only a basic lab set-up. Although the method of chemical synthesis is simple, the particle dimension distribution of particles generated using this method is poly-dispersed. Furthermore, the processing of hydrogen gas during synthesis poses a safety risk, necessitating careful handling and the use of explosion-proof mixers (Yirsaw et al., 2016). Several researchers have reported the synthesis of nZVI using the reductive effect of sodium borohydride (NaBH4) on ferric chloride (FeCl3⋅6H2O) (Bae et al., 2016; Eljamal, Eljamal & Khalil, 2017; Junying et al., 2011; Mao & Gregory, 2015) ever since its discovery (Brown & Subba, 1955). The hydrazine method, albeit a not so popular technique, is also used in the production of nZVI (Pasinszki & Krebsz, 2020). Sodium borohydride and hydrazine hydrate methods appear to be among the simplest reduction methods used in the synthesis of metal nanoparticles (Jamkhande et al., 2019); however, there are many drawbacks to reducing agents, including toxicity, cost, poor reducing capability and impurities (Zhang et al., 2010). In an experiment, hydrazine hydrate was employed as a reducing agent (Halder et al., 2021; Rai et al., 2021; Veisi et al., 2021; Vilardi, Verdone & Bubbico, 2021) to anhydrous FeCl3 in the synthesis of Fe nanoparticles where polyethylene glycol (PEG) and carboxymethyl cellulose (CMC) acted as stabilizers (Parimala & Santhanalakshmi, 2014).
Semi-green route (sodium dithionite)
Under high pH and in the absence of oxygen, dithionite can be used to reduce Fe(II) and produce nZVI. In a thin platelet, the nZVI is coprecipitated with a sulfite hydrate. Although the nanoparticulate materials produced are usually unclean Fe, it makes sure that their ability to degrade in the presence of air or N2 is not impaired. When using nanoparticles made from dithionite, the quality of trichloroethylene (TCE) degradation is comparable to that of the more traditional borohydride method. The dithionite approach also has the following advantages: (i) it makes use of a reducing agent that is less costly and readily available; (ii) there is no output of hydrogen gas, which may be explosive. As benzoic acid is oxidized with nZVI–dithionite particles, different less toxic byproducts are generated than when nZVI–borohydride particles are used. The processing of higher concentrations of phenol compensates for the low oxidant yield dependent on hydroxybenzoic acid generation. When using nZVI-dithionite particles, the high phenol concentration compared to hydroxybenzoic acids shows that OH radical incorporation is not the main oxidation pathway. It is suggested that hydroxyl radical attack on the sulfite matrix surrounding the nZVI–dithionite particles produces sulphate radicals (SO4.−), and by using electron transfer reactions rather than addition reactions, these radicals oxidize the benzoic acid (Parimala & Santhanalakshmi, 2014; Pasinszki & Krebsz, 2020; Quan et al., 2008).
Green route (plant extract) for zero-valent iron nanoparticles synthesis
Zero-valent iron nanoparticle can also be synthesized using a plant extract (Tarekegn, Hiruy & Dekebo, 2021). The iron nanoparticles obtained with this method were found to efficiently remove toxicants like Cr (VI), as investigated by Fazlzadeh et al. (2017). The plant extracts used in this study were Rosa damascene (RD), Thymus vulgaris (TV), and Urtica dioica (UD). Compared to the chemical nanoparticle synthetic methods, polyphenols, proteins, and organic acids, which act as reducing and stabilizing agents, minimize the risk of nanoparticle aggregation. The composition and agglomeration of nanoparticles after adsorption are affected by the volume and form of compounds in plant extracts (Fazlzadeh et al., 2017). The performance of mulberry and oak leaves synthesized zero-valent iron nanoparticle, while immobilizing Ni and Cu in polluted sediment was also reported (Slijepcevic et al., 2021).
Stabilized zero-valent iron nanoparticles synthesis
Magnetic attraction that occurs between iron nanoparticles influences the rapid aggregation of particulate materials (Awang et al., 2023; Chatterjee, Lim & Woo, 2010), and as such a number of organic coatings, e.g., polymers, mixtures (i.e., emulsions), and poly-electrolytes, are utilized nowadays, which limits reactiveness yet boosts the movement of iron nanoparticles (Meunier et al., 2006). Pure as well as exposed nano zero valent iron has the likelihood of reacting to disbanded oxygen as well as oxygen species. Therefore, it is necessary to cover it using surfactants, otherwise with poly-electrolytes. Nanoparticles modified using some particular poly-electrolytes were found to demonstrate more mobility when compared to exposed nano zero valent iron for a short as well as for a long period of time. Cirtiu et al. (2011), were able to prove how particles can be stabilized and continue to be mobile when covered with some particular poly-electrolytes (e.g., polystyrene sulfonate, carboxymethyl cellulose, poly-acrylic acid and poly-acrylamide) and last through 8 months or longer following the initial introduction based upon geochemistry as well as hydrochemistry discovered at location (Cirtiu et al., 2011). In a research conducted by Ponder, Darab & Mallouk (2000) and Ponder, Darab & Mallouk (2000), starch was used to stabilize iron nanoparticles for a better reactivity and mobility for Cr (VI) removal. Various surface coatings can be utilized for the improvement of zero-valent iron nanoparticle stability.
-
Chitosan
-
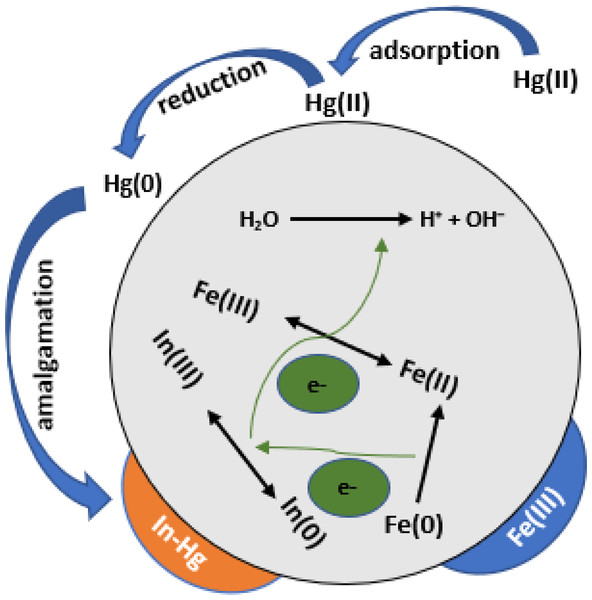
Indium (In) (Fig. 4), describes the formation of nano/micro structures on nano zero-valent iron (Xia, Ling & Zhang, 2017).
-
Polyelectrolytes like ion-exchange resins, block copolymers and polyacrylic acid.
-
Amphiphiles together with several surfactants and block copolymers.
-
Numerous oil-founded micro-emulsions.
Characterization of zero-valent iron nanoparticles
The structure as well as phase of the synthesized ZVI nanoparticles are analyzed by means of X-ray diffraction. The elemental composition is determined using energy-dispersive X-ray spectroscopy. The surface (or up to less than 10 nm-depth of the nanoparticle) is obtainable using XPS (X-ray photo-electron spectroscopy) which determines the number of species present on the surface. XANES (X-ray absorption near edge structure) provides valuable info with regards to ionic valency. The zeta potential (z) is used frequently to characterize the charge on ironic nanoparticles’ surface. The surface area and pore-size analysis are performed on the Brunauer–Emmett–Teller (BET) surface area analyzer (Hwang, Kim & Shin, 2011).
Figure 4: Hg (II) elimination by In-ZVI particulate material under oxygen conditions is depicted in a schematic diagram (Qasim et al., 2020a; Qasim et al., 2020b).
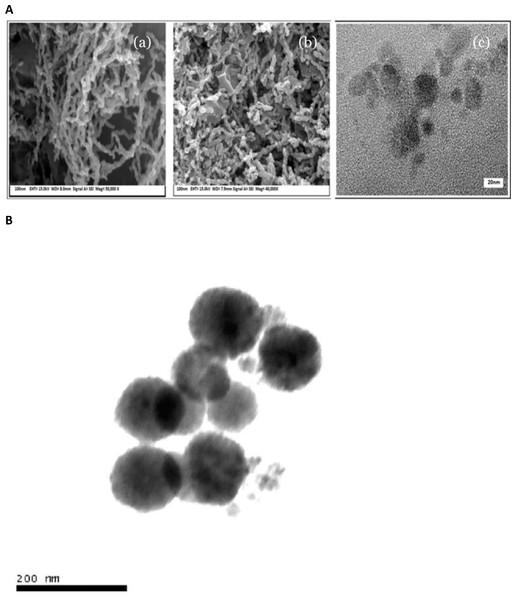
The morphological examination is carried out using Field Emission Scanning Electron Microscope (FESEM) and Transmission Electron Microscope (TEM) analysis, an illustration of such is given in Figs. 5A and 5B (Li, Elliott & Zhang, 2006; Shaikh et al., 2022). The sample formulation is to be achieved by placing two or three dribs of diluted mixture of ethanol of the sample to a copper (300-mesh)-supported film of carbon surface. The sample is to be placed in a vacuum cover until the ethanol is fully evaporated. The electrostatic and magnetic interactions cause the particles to aggregate which are obtained as clustered nanoparticles making sequence structures, as revealed in FESEM and TEM analysis. An Acoustic Spectrometer is utilized to measure the distribution dimension and particle dimension of nZVI using transmissible pulses of sound via particulate suspension, in addition to determining the characteristics of particles in suspension (Yan et al., 2010).
Figure 5: (A) Zero valent iron nanoparticle; (A–B) FESEM images and (C) TEM image, and (B) TEM images (200 nm) of nZVI synthesized using NaBH4 reduction of FeCl3 method.
Reproduced with permission from Li, Elliott & Zhang (2006) and Shaikh et al. (2022). ©Taylor & Francis.Mechanism of zero-valent iron nanoparticles action
Until recently, precipitation by chemical means (Matlock, Howerton & Atwood, 2002; Meunier et al., 2006; Román-Ross et al., 2006), electrochemical procedure, electro dialysis (Meunier et al., 2006), recovery by evaporation (Bouhamed, Elouear & Bouzid, 2012), extraction of solvent (Li et al., 2012), ultra-filtration (Zondervan & Roffel, 2007), ion-exchange (Oehmen et al., 2006; Azarudeen et al., 2013), reduction and oxidation processes, (Ding et al., 2015; Ramos et al., 2009; Sun, Li & Wang, 2014), reverse osmosis (Akin et al., 2011), filtration (Leupin & Hug, 2005), adsorption (Ding et al., 2015; Mukherjee et al., 2016; Ren et al., 2014; Sun et al., 2014; Sun et al., 2015; Sun et al., 2016a; Sun et al., 2016b; Wang et al., 2019; Zhao et al., 2014) and membrane (Gao et al., 2014; Zondervan & Roffel, 2007) technologies have been the most widely used approaches towards removing toxic heavy metal ions from unclean water. Conversely, many of these methods experience several challenges; for example, chemical precipitation, although reliable, requires high installations at some expense because of its huge reservoirs in order to obtain the effectual precipitation (Matlock, Howerton & Atwood, 2002; Yan et al., 2010).
Remediation of polluted sites necessitates the implementation of a cost-effective, quick, and environmentally safe process. As nanoparticulate zero valent iron draws considerable attention in environmental remediation (Li, Elliott & Zhang, 2006; Zhang, 2003), its application in transforming a broad array of environmental pollutants like heavy metals, chlorinated hydrocarbons, pesticides, nitrate, etc. has comprehensively been recognized and recorded (Elliott, Lien & Zhang, 2009; Ponder, Darab & Mallouk, 2000; Varanasi, Fullana & Sidhu, 2007).
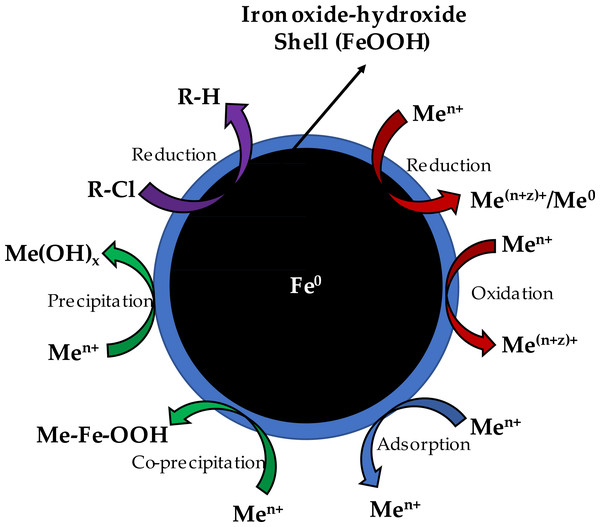
The nZVI contains a core–shell in its structure which allows it to express the properties of an electron source offered by the core, as well as a complexation surface provided by the shell. The principles of nZVI’s reaction with metals involves the ability of the Fe0 in the structure to reduce the metals while allowing the ferric oxide shell to interact electrostatically and reactively with the heavy metals. The mechanism by which nZVI interact with metals can be grouped into several classes (Pasinszki & Krebsz, 2020; Yang et al., 2019):
Figure 6: Schematic diagram of Zero-valent iron nanoparticle structure illustrating removal mechanism of heavy metals, organic/inorganic, waste sludge and other toxic compounds (Yang, Kung & Chen, 2019).
-
Adsorption, (e.g: U, As, Pb, Co, Ni, Cr, Cd, Ba, Zn, and Se ions)
-
Co-precipitation (e.g: Cr, As, Ni, and Se ions)
-
Reduction (e.g: Pb, Pd, Pt, Hg, As, Ag, U, Se, Cu, Co, Cr, and Ni, ions) and
-
Precipitation (e.g: Co, Zn, Cu, Cd, and Pb ions)
Fig. 6 shows nZVI’s core–shell structure, illustrating the reduction and adsorption mechanisms of heavy metals. When using nZVI for heavy metal removal, it is important to note that there exists a difference in mechanism for the removal of different heavy metals caused by their standard potential E0 (Table 2 lists the E0 of certain environmentally important metals (Li & Zhang, 2007). Heavy metal ions are characteristically reduced if their E0 is higher than the E0 of Fe2+/3+ or Fe (e.g., Hg, Se, Cr, As, Cu, Ag, and U ions), making the form that was reduced to be precipitated either near to or on nZVI surface. The E0 of metal ions like Ni and Pb ions are a bit higher than the E0 of Fe2+/3+ or Fe, therefore they may be adsorbed and equally reduced. In another instance, metal ions Zn2+ and Cd2+ possess less E0 than Fe2+/3+ or Fe and are predominantly adsorbed onto hydroxide/oxide or topping coating of the nZVI, thereby not risking their reduction (Pasinszki & Krebsz, 2020; Yang et al., 2019). Both reduction and adsorption mechanisms can be expressed in the equations below: (1) (2)
| Metal | E0(V) | |
|---|---|---|
| Barium (Ba) | Ba2+ + 2e −↔ Ba | −2.90 |
| Zinc (Zn) | Zn2+ + 2e −↔ Zn | −0.76 |
| Iron (Fe) | Fe2+ + 2e −↔ Fe | −0.41 |
| Cadmium (Cd) | Cd2+ + 2e −↔ Cd | −0.40 |
| Nickel (Ni) | Ni2+ + 2e −↔ Ni | −0.24 |
| Lead (Pb) | Pb2+ + 2e −↔ Pb | −0.13 |
| Copper (Cu) | Cu2+ + 2e −↔ Cu | 0.34 |
| Silver (Ag) | Ag+ + e −↔ Ag | 0.80 |
| Mercury (Hg) | Hg2+ + 2e −↔ Hg | 0.86 |
| Chromium(Cr) | Cr2O72− +14H+ + 6e −↔ 2Cr3+ +7H2O | 1.36 |
Reductive removal of mercury using zero-valent iron nanoparticles
Metals such as Ag, Hg, Cu, and Pb, which have a considerably higher standard potential (E0) than iron, are mainly extracted by reduction (Liang et al., 2021), while those with a potential (E0) similar or negative to iron (e.g., Cd, Zn) are often eliminated by adsorption. Table 3 summarizes some of the different nZVI adsorbents used for the removal of mercury since 2010. In these studies, as represented in the table, the form in which the nano iron exists, concentration of mercury, the optimal condition, sorption isotherm used and of course the actual remediation location determine the removal efficiency of mercury in the targeted remediation zone. The studies also displayed how functionalized—nZVI is effective and increases the ability of nZVI towards the reduction and removal of mercury. ZVI oxidation to ferrous iron can occur as anticipated by the large negative free energy transition shown in Eq. (3), which can lead to divalent Hg(II) reduction to Hg0 (Qasim et al., 2020a). (3) (4) (5)
| Form of nZVI | Mercury tested (mg/L) | Sorption isotherm | Optimal conditions | Remediation of actual wastewater | Mercury removal | Ref. | |
|---|---|---|---|---|---|---|---|
| % | mg/g | ||||||
| Raw | |||||||
| nZVI | 10.03 | Not applicable | pH 5, temp 25 °C | Wastewater sediments | 75 | – | Chapman, Moore & Campbell (2020) |
| Magnetite (Fe3O4) | 100 | Not applicable | Temp 25 °C | Wastewater containing mercury | <40 | 28 | Inglezakis et al. (2020) |
| Functionalized | |||||||
| Polythiophene modified chitosan/magnetite nanocomposites | 100 | Freundlich & Langmuir | pH 7, temp 25 °C | Aqueous solution | – | 51 | Morsi et al. (2018) |
| Dithiocarbamate–Fe3O4 | 50 | Langmuir Freundlich, Sips and Redlich-Peterson | pH 7, temp 25 °C | Natural spiked waters | 99.8 | 206 | Figueira et al. (2011) |
| Zeolite–Fe3O4 composite | 700 | – | Temp 25 °C | Wastewater containing mercury | – | 26.2 | Andrade et al. (2019) |
| Fe3O4-Ag0 | 100 | Not applicable | Temp 25 °C | Wastewater containing mercury | >80 | 71.3 | Inglezakis et al. (2020) |
| Fe3O4/γ-Fe2O3–Aloe vera supported | 8.0 | – | pH 11–12, temp 25 °C | Wastewater containing mercury | 87 | – | Vélez et al. (2016) |
| Graphene oxide-iron magnetic nanoparticle (GOIMNP) | 50 | Elovich model | pH 5, temp 25 °C | Aqueous solution | – | 16.6 | Diagboya, Olu-Owolabi & Adebowale (2015) |
| Sulfidated nanoscale zero-valent iron (S-nZVI) | 750 | Not applicable | Temp 25 °C | – | 99 | – | Liang et al. (2021) |
| Ostrich bone ash supported nano zero-valent iron (OBA-nZVI) | 100 | Not applicable | pH 7, temp 25 °C | Natural surface and ground water | 96.7 | – | Amiri, Abedi-Koupai & Eslamian (2017) |
| Glutathione binded-nZVI | 60.0725 | Not applicable | pH 5, temp 25 °C | Wastewater containing mercury | 99 | – | Qasim et al. (2020b) |
| Pumice–nZVI | 100 | – | pH 8.13, temp 25 °C | Wastewater containing mercury | – | 332.4 | Liu et al. (2014) |
| Magnetic beads–nFe3O4 | 5.0 | Langmuir | Temp 34 °C | Developed to remove mercury from human blood | – | 6.49 | Okamoto et al. (2011)) |
| Magnetic activated carbon–nFe3O4 | 10 | Langmuir | Temp 34 °C | Developed to remove mercury from human blood | – | 38.3 | Okamoto et al. (2011) |
| Magnetic nanoparticles–amino propyl silane (MNP–APS) | 100 | Langmuir and Freundlich | pH 8, temp 25 °C | Aqueous solution | 94 | – | Marimón-Bolívar, Tejeda-Benítez & Herrera (2018) |
| Magnetic nanoparticles–Peptone | 100 | Langmuir and Freundlich | pH 7, temp 25 °C | Aqueous solution | 87 | – | Marimón-Bolívar, Tejeda-Benítez & Herrera (2018) |
| Magnetic nanoparticles–yam peel biomass (MNP–YP) | 100 | Langmuir and Freundlich | pH 7, temp 25 °C | Aqueous solution | 75 | – | Marimón-Bolívar, Tejeda-Benítez & Herrera (2018) |
| Silica–Fe3O4 | 0.05 | – | Temp 25 °C | Wastewater containing mercury | 74 | – | Girginova et al. (2010) |
| Thiol–magnetic nanoparticles (TF-MNP) | 300 | Langmuir and Freundlich | pH 6, temp 25 °C | Aqueous solution | – | 344.82 | Oveisi et al. (2017) |
| Poly(1-vinylimidazole)–Fe3O4@SiO2 magnetic nanoparticle | 1.0 | Freundlich and Langmuir | pH 7, temp 25 °C | Water containing mercury | >94 | 346 | Shan et al. (2015) |
| Ostrich bone ash/nZVI composite | 5–1000 | Langmuir and Freundlich | pH 1–9, temp 25 °C | Aqueous solution | – | 170 | Gil et al. (2018) |
| Pumice–nZVI | 1.5 uM | Freundlich | pH 7, temp 25 °C | Oxic/anoxic solution | – | 6.1/1.5 | Qasim et al. (2018) |
| Magnetic iron oxide–2-mercaptobenzothiazole | 50 ng mL−1 | Langmuir | pH9, temp 25 °C | Contaminated surface water | 98.6 | 0.125 | Parham, Zargar & Shiralipour (2012) |
| CoFe2O4@SiO2@m-SiO2-SH/NH2 | 40 | Langmuir | pH 7.2, temp 35 °C | Aqueous solution | – | 517.4 | Zhang et al. (2020) |
| Puffed rice carbon-Fe-Sulfur (PRC-Fe@S) | 300 | Langmuir | pH 6, temp 25 °C | Aqueous solution | – | 738.0 | Fang et al. (2020) |
| Mercaptoamine-functionalised silica-coated magnetic nanoparticles | 50 | Freundlich | pH 5–6, temp 25–45 °C | Wastewater containing mercury | – | 10 | Bao et al. (2017) |
| Alloy with metals | |||||||
| FeS | 1.0 | Freundlich | pH 7, temp 30 °C | Aqueous solution | >96% | 769.2 | Sun et al. (2017) |
| Pyrite | 1.0 | Langmuir | pH 7, temp 30 °C | Aqueous solution | >96% | 9.9 | Sun et al. (2017) |
| In–nZVI | 216.59 | Langmuir | pH 7, temp 25 °C | Aqueous solution | >99 | – | Qasim et al. (2020a) |
A new technique was used in removing mercury (II) from wastewater by pumice-enhanced zero-valent iron nanoparticle composite (P-nZVI) prepared using liquid-phase technique through reducing FeCl3.6HO2 with NaBH4, and the removal capacity was found to be high (Liu et al., 2014). In another investigation, the effects of dissolved oxygen and nitrate on the reduction and removal of Hg(II) by P–nZVI were studied, in which the maximum removal of Hg(II) in both oxic and anoxic aqueous solutions were 6.1 mg/g and 1.5 mg/g, respectively (Qasim et al., 2018). Liang et al. (2021) were able to remove Hg(II) ion using sulfidated zero-valent iron nanoparticle (S-nZVI) and by using bare nZVI. A one-step liquid technique was used to synthesize S-nZVI, and in 5 min more than 99% of Hg(II) was removed by both S-nZVI and nZVI after measuring the ionic concentration (Liang et al., 2021).
An investigation into the effect of glutathione (GSH) on nZVI and pumice-supported nZVI in the presence of natural organic matter was conducted by Qasim et al. (2020b). In this study, pumice-supported nZVI was also synthesized using NaBH4 as reductant to reduce mercury. The reduction process in the presence of natural organic matter from the Suwannee River revealed that there is a decreasing trend in mercury removal from 89 to 36 percent in 80 mins. However, the trend tends to increase from an 85 to 96 percent removal after the addition of GSH to nZVI (without Suwannee natural organic matter) in the following 15 days. Moreover, Hg(II) removal efficacy increased greatly, following the addition of GSH to nZVI in the river’s natural organic matter, to more than 99 percent in 9 days. In another instance, Fang et al. (2020) developed a high-quality PRC/Fe@S composite that efficiently adsorbed mercury. Fe nanoparticles formed in situ are closely combined with sulphur and then homogeneously scattered using a supercritical CO2 fluid approach, forming an architecture which is a porous hierarchical cross-linked structure with abundant pores and voids for absorbing Hg(II). The PRC/Fe@S composite shows high removal capacity, superior selective affinity, and an ultrahigh adsorption capability of up to 738.0 mg/g. The hierarchical porous carbon in the PRC/Fe@S composite also acts as a structure that stabilizes and efficiently disperses Fe nanoparticles. More specifically, the absorbed Hg(II) can be chemically immobilised by sulphur after Fe reduces it to Hg0 (Fang et al., 2020).
More experimental work on the removal of Hg(II) by ostrich bones-supported nZVI was also documented (Amiri, Abedi-Koupai & Eslamian, 2017). A prepared ostrich bone ash solution was made to react with FeCl2.4H2O where NaBH4 acted as the reducing agent. The immobilized form of ostrich bone ash supported-nZVI was obtained after filtration. A fixed-column bed experimentation was utilized, and samples of effluent were used at different times until adsorbents’ saturation was reached, and mercury ions’ removal was able to achieve 99.7%. Another investigation of mercury removal was done using indium-doped ZVI (In-ZVI). To maximize Hg(II) reduction and removal reactivity and longevity, zero-valent iron was impregnated with In, and the removal percentage was found to be 99.3 following measurement of Hg’s removal efficiency. Following seven consecutive spikes of Hg(II), no substantial Hg(II) decrease in removal efficiency was found in the longevity test of In-ZVI, which gave a removal of 99.9% during the first spike as well as delivering 99.4% removal during the seventh spike (as seen in Fig. 4 above). Various control experiments revealed that the pairing of oxidation of In0 and reduction of Fe(III) that surfaced on In-ZVI obviously resulted in the production of atomic reactive hydrogen (H.), leading to an increase in the reactivity of ZVI as well as longevity in the reduction and extraction of Hg(II) (Qasim et al., 2020a).
Removal of mercury using iron-based adsorbents
Adsorbents of iron-based ferric can also be used in the reductive removal of mercury. Such adsorbents may include iron oxide nanoparticles, maghemite (Fe2O3), magnetite (Fe3O4), and iron oxide nanocomposites. Nanocomposites of different materials with iron oxide have been used in mercury removal from water. Due to their nontoxic nature, being less costly, easily applicable and also able to be separated easily from solution aqueous in nature following the application of a magnetic field, nanocomposites of magnetite have been investigated broadly for water treatment. It has also been used as a core that is encased in a silica membrane (Inglezakis et al., 2020). Table 4 shows different magnetite-based nanocomposites and their maximum capacity for mercury removal.
| Nanocomposite | Max. mercury removal (mg/g) |
|---|---|
| Fe3O4-SiO 4–poly(1-vinylimidazole) | 346 |
| Fe3O4–Silica shells–Dithiocarbamate | Satisfactory |
| Fe3O4–Chitosan–polythiophene | 50 |
| Fe3O4–Thiol | 345 |
| Fe3O4–Amino organic ligands–yam peel biomass | 60 |
| Fe3O4–Dithothreitol | 6.3 |
| Fe3O4–Activated carbon doped | 38.3 |
| Fe3O4–Dithiocarbamate | 122–246 |
| Fe3O4–Zeolite | 26.2 |
Maghemite (γ–Fe2O3) and magnetite (Fe3O4), which were stabilized with Aloe Vera, were used in the removal of Hg(II) (Vélez et al., 2016). The average size of the iron oxides nanoparticles synthesized was ∼100 nm and more than 85% Hg(II) removal capacity with an average removal capacity of 70% attained. It was also reported that Fe3O4, functionalized with poly(2–aminoethyl methacrylate) hydrochloride polymer chain and Dithiocarbamate groups, was able to completely remove Hg(II) from water as it possesses great chelating affinity towards mercury (Dave & Chopda, 2014). Nanoparticulate magnetic iron oxide, when modified with 2–mercaptobenzothiazole, attained a maximum adsorption capacity of 98.6% for 50 ng/mL Hg(II) ion concentration compared to 43.47% maximum adsorption capacity without modification using the same 50 ng/mL concentration of Hg(II) ions (Parham, Zargar & Shiralipour, 2012). At a temperature of 80 °C and pH 7.2, Hg(II) ions were adsorbed by an enhanced bifunctional group and core shell magnetic nanoparticulate adsorbent (CoFe2O4@SiO2@m-SiO2-SH/NH2). In this study, the maximum mercury ion removal capacity was found to be 517.4 mg/g (Zhang et al., 2020). Moreover, Bao et al. (2017) synthesized an effective silica–coated mercaptoamine–functionalized nanomaterial and was used in the extraction of mercury from wastewater. In 120 min, an equilibrium was attained and the maximum adsorption was found to be 10 mg/g of Hg(II) at pH 5–6. The ion exchange process between the heavy metal ions and thiol functional groups and chelation through the amine group on the nano-adsorbent surface is most likely involved in the process of sorption as was assumed by this investigation (Bao et al., 2017).
In another study, graphene oxide magnetic nanoparticle (GOMNP) was synthesized by way of a co-precipitation method and used in the adsorption of Hg2+. A pre-adsorption procedure was done with graphene oxide which was found to have less adsorption capacity for mercury. Interestingly, the adsorption kinetics of the graphene-oxide magnetic nanoparticle at the end of the analysis showed a faster adsorption and a more than 5-fold higher capacity for mercury adsorption within 120 mins (Diagboya, Olu-Owolabi & Adebowale, 2015). Additionally, in a quest to develop highly efficient adsorbents, tremendous efforts were put in place in producing doped materials. A novel nanostructured biomagnetite Zn-doped material, Zn0.46Fe2.54O4, was created. Because of its simpler action in the aqueous method of separation, a modified 3-mercaptopropyl trimethoxysilane (MPTMS)–Zn0.46Fe2.54O4 offers hopes for the removal of Hg(II) as a result of its outstanding adsorption capacity for Hg(II) in real applications (Yu et al., 2016). Gil et al. (2018) were able to come up with a highly efficient technique for the enhancement of nZVI using ostrich bone ash (OBA), and produced OBA–nZVI as a novel adsorbent, which was then used for Hg(II) ions’ removal from aqueous solutions. A maximum adsorption capacity of 170 mg/g of Hg(II) ions was attained using 20% loading amount of iron (Gil et al., 2018).
Factors affecting the performance of zero-valent iron nanoparticles
For the last two decades, iron nanoparticles have seen new usage opportunities as a permeable reactive defense (a few meters in length) (Astrup, Stipp & Christensen, 2000; Blowes, Ptacek & Jambor, 1997; Blowes et al., 2000; Yirsaw et al., 2016). The list of chemicals remedied using iron nanoparticles is increasing and includes pesticides, bromates, nitrates, nitroaromatic compounds, chlorates, chlorinated organic compounds, lead, asbestos, and hexavalent chromium—all of which have been remedied at the laboratory and field scale. Since the size of the particles affects nZVI’s reactivity, other variables that may affect their performance include passivation, geochemical processes, and agglomeration (Yirsaw et al., 2016).
(i) Geochemical process
Fe0 is a good source of reducing alternatives, and it can also be used as an electron donor, namely Fe2+ or H2 Eq. (6) (6) This is where ΔG0 and E0 respectively represent the Gibbs free energy and reduction potential. Conversely, in procedures involving geochemical as in a reaction involving chemicals in water or soil, the number of alternatives to reduction of nZVI available for use in reactions with intended pollutants would be reduced (O’Carroll et al., 2013). The remediation efficiency of nZVI is affected by numerous parameters including the ionic strength of the groundwater, the composition of the soil matrix, and geochemical properties such as dissolved oxygen, pH, and oxidation reduction potential (ORP) (Yueqiang & Lowry, 2006). Thus, before injecting nZVI, a thorough understanding of the geologic and hydrogeologic conditions is needed for better results.
(ii) Passivation
In practice, nZVI are subjected to corrosion, with corrosion materials and other precipitates, e.g., goethite, maghemite, iron (III) hydroxides, lepidocrocite, and hematite coating the iron surface. nZVI can be passivated with the loss of redox reactivity when exposed to oxygen and other oxidants, leading to restriction of electron transfer and hydrogen formation reactions, where the use of a volume of water infused along with the iron would limit the function of nZVI. As a result of such passivation, the handling rate of targeted pollutants will be reduced. The production of mixed valent iron oxides, such as magnetite, on the other hand, would speed up the remediation process (Yirsaw et al., 2016).
(iii) Agglomeration
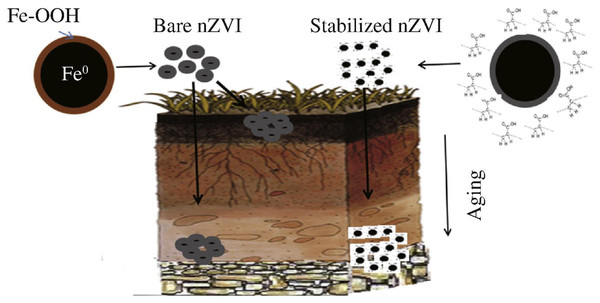
Another drawback is that because of the accumulation and connection to or deposition on aquifer surfaces, In water-saturated porous media, zero-valent iron nanoparticles have limited mobility (Fig. 7) (Phenrat et al., 2007). This may be attributed to the particle’s physical characteristics, e.g., dimension, zeta potential as well as concentration of application and Brownian motion are included (Phenrat et al., 2007; Phenrat et al., 2009). As a function of Brownian motion, random collisions of particles in the atmosphere are continuous. Agglomeration happens when the energy of attraction for the surfaces of soil grains surpasses the energy of repulsion (Tourinho et al., 2012).
Figure 7: Behavior of bare and stabilized nZVI in the environment.
Reproduced with permission from Yirsaw et al. (2016). Copyright 2016 Elsevier.Based on another finding, it was reiterated that nZVI reactivity, along with its utility for remediation of the environment, are hampered by three major issues: immobilization, agglomeration, and passivation. Immobilization involves the stabilization of nZVI in a liquid or solid medium’s hard structure (e.g., via the processes of sedimentation or sorption). Agglomeration, on the other hand, displays the manner in which zero-valent iron nanoparticles are assembled to establish a larger particle dimension, which reduces their usefulness in water and as such weakens their strong reactive surface area. To be more precise, these nanoscale particles, specifically original nZVI, used to be agglomerated as well as delivering capable stabilized particles of micrometer dimensioned. When that occurs, nZVI may take on the characteristics of larger natural colloids, resulting in a lack of nanoparticle properties. Moreover, passivation can occur via nano zero-valent iron oxidation prior to achieving the pollutants it was intended to react on. Passivation can also occur as a result of the rapidity of nano zero-valent iron oxidation due to the available oxygen of the medium and agglomeration. Additionally, aside from the aforementioned, the dangers associated with nZVI handling pose a threat to the community. People employed with nZVI are at risk when transporting, treating, and injecting nanoparticle slurries for in-situ applications (Ken & Sinha, 2020).
Toxicity of zero-valent iron nanoparticles
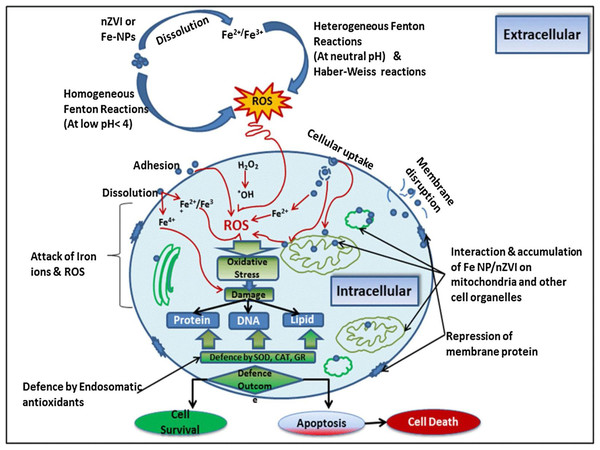
Nano zero-valent iron is a promising emerging technology due to its considerable surface area, relative cost effectiveness, ease of transportation, easily adoptable, prominent reductive nature, and suitability for in-situ applications when compared with other technologies (Ken & Sinha, 2020). However, iron nanoparticles have been shown to exert a variety of toxicological effects on biochemical processes. According to some research, ions emitted from metal nanoparticles can cause an overabundance of free radicals and reactive oxygen species (ROS), causing oxidative harm in organisms. These highly reactive oxygen free radicals have the ability to oxidize proteins, lipids, and DNA in the cell (Fig. 8). Furthermore, free metal ions can influence bacterial species by interfering with enzyme activity and integrity of the cellular membrane (Daraei et al., 2019).
Figure 8: Potential harmful mechanism and association of nanoscale zero-valent iron (Fe-NPs) with living organism’s cells.
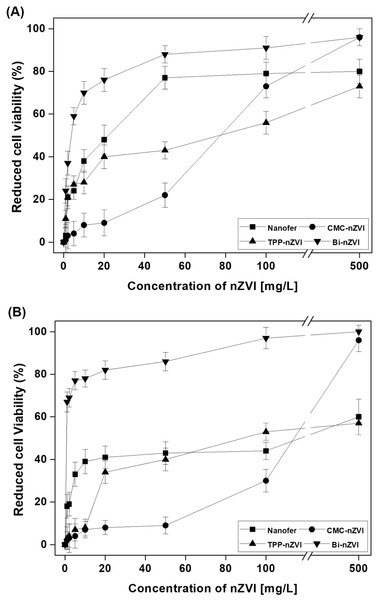
Reproduced with permission from Ken & Sinha (2020). Copyright 2020 Elsevier.Having known the toxic effects of the ZVI nanoparticle, there is however less knowledge about the toxicity of surfaced-modified nano zero-valent iron on different species in the ecosystem. Yoon et al. (2018) performed an investigation in which they compared the toxicities of bare nanoscale zero-valent iron (nanofer), carboxymethyl cellulose (CMC)-stabilized nanoscale zero-valent iron, tetrapolyphosphate (TPP)-coated nanoscale zero-valent iron, and bismuth (Bi)-doped nanoscale zero-valent iron on a variety of organisms, both terrestrial and aquatic, which include bacteria (Escherichia coli and Bacillus subtilis), plant (Arabidopsis thaliana), earthworm (Eisenia fetid a) and water flea (Daphnia magna). The bismuth and CMC-nanoscale zero-valent iron induced opposing biological reactions in all the tests done apart from E. fetida, changing from cell death in B. subtilis and E. coli to physiological inhibition in A. thaliana and D. magna. The surface alteration of nano zero-valent iron played a major role in their toxicities by modifying their physico-chemical properties according to particle characterization under exposure conditions. nZVI can cause toxicity through several routes such as promotion of oxidative stress, cell membrane destruction, chlorosis, or hypoxia. Figure 9 shows the reduced cell viability of B. subtilis and E. Coli and when exposed to the bare and surface-modified nano zero-valent irons.
Figure 9: E. coli. (A) and Bacillus subtilis (B) viabilities following nano zero-valent iron test in deionized water for one day.
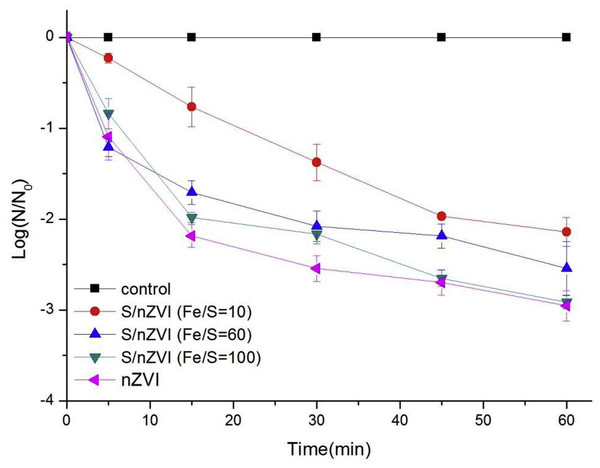
Reproduced with permission from Yoon et al. (2018). Copyright 2018 Elsevier.Nanoscale zero-valent iron in water can easily inactivate E. coli, as well as the activity of bacteria that was also seen in other iron-based compounds (Liang et al., 2019). The toxic effect of sulfide-modified nZVI on E. coli in aqueous solutions was also studied by Cheng et al. (2019). The surface sulfide modification (that can reduce nano zero-valent iron toxicity) and sulfide-modified nZVI at lower molar ratio of Fe/S displayed weaker toxicity due to lesser Fe0 and higher sulfate and iron oxide contents. Figure 10 shows toxicity assessments of sulfide-modified nZVI on E. Coli that were conducted at 3 different Fe/S molar ratios.
Figure 10: NZVI and S/nZVI with 3 different Fe/S molar ratios with time inactivate E. coli (Conc of nZVI or S/nZVI: 10 mg /L).
Reproduced with permission from Cheng et al. (2019). Copyright 2019 Elsevier.The effects of nano zero-valent iron have been quantified by different studies. Elongation of the root was triggered by Arabidopsis thaliana when exposed to the ZVI nanoparticle, likely because of ZVI nanoparticle’s exceptional redox properties. Zero valent iron nanoparticle’s concentrations of 500 mg/L or less make it possible to increase the biomass of different species of plant like perennial ryegrass, rice and peanut. However, higher concentrations of the zero-valent iron nanoparticle of more than 1,000 mg/L inhibited cattail growth, rice, and cotton wood plants. These explain why an optimal nanoparticulate zero-valent concentration may be required to promote the growth of plants. On the other hand, it should be noted that the effects of the zero-valent iron nanoparticle are dependent on species, while explanations as to why it leads to an increase in toxicity and biomass in plants are yet to be understood (Yoon et al., 2019).
A high amount of zero-valent iron nanoparticle, e.g., >100 mg/L, inadvertently emitted from in situ places to adjacent oxygenated aquifers can be oxidized quickly to oxides of iron (e.g., Fe3O4 or Fe2O3) as well as ions (e.g., Fe2+), causing acute hypoxia in the marine environment. The oxidation products and ecotoxicological fate of zero-valent iron nanoparticle as well as environmental surface water concentrations (considering waterborne conveyance or waste expulsion) in relation to introduction to marine vertebrate species still remain unknown. Yang, Kung & Chen (2019) conducted a study to assess the unpremeditated effect of carboxymethyl cellulose-stabilized zero-valent iron nanoparticle (CMC-nZVI), Fe2+ and nanoparticulate iron oxide (nFe3O4) on medaka adult fish for reproductive toxicity. The introduction was done for 21 days at Fe equivalent of 5 and 20 mg/L (Fig. 8). These concentrations did not alter the redox potential nor dissolved oxygen or values of the pH in the iron solutions at the introduction time. None of the treated medaka adult fish showed alteration responses in regular production of egg and oxidative stress in tissues observed. However, the production of egg in nanoparticulate iron oxide-treated pairs with 20 mg/L concentration was reduced, with higher occurrence of irregular undeveloped oocytes in the ovary. In the ovary and brain, activities of superoxide dismutase and glutathione peroxidase expression were suppressed by nanoparticulate iron oxide treatment. Despite the inhibition of the expression of mRNA in the hepatic estrogen receptor in females due to iron oxide nanoparticle or Fe2+ treatments, the levels of plasma of sex hormones as well as activity of (Na-K)-ATPase in gills of medaka fish do not change when compared with both sexes. As a consequence, by activating the oxidative stress in the female gonads, products of oxidation from nano zero-valent iron at lower milligram-per-liter amounts can be successful in persuading nanoparticle-specific reproductive toxicity in medaka fish (Yang, Kung & Chen, 2019).
To reduce the toxicity of nZVI, there needs to be an optimal concentration for both terrestrial, aquatic and bacterial environment. For example, as Yoon et al. (2019) reported, 500 mg/L (and less) of nZVI increase the biomass in plants, while a concentration of more than 1,000 mg/L inhibit growth (Yoon et al., 2019), and similarly a concentration greater than 100 mg/L could cause acute hypoxia in marine life which was pointed out by Yang, Kung & Chen (2019). There should be an optimum value to suit each environmental condition. Surface-modified nanoscale zero-valent iron appears to be less harmful even in a high amount to microorganisms and bacteria. However, the choice of stabilizing material is paramount towards reducing nZVI-surface modified toxicity to bacteria and microbes. For instance, in the work of Liang et al. (2019), Escherichia coli activity with bare nZVI appears lower than with biochar- surface modified and less toxic.
However, some reports demonstrate the possible benefits of nZVI despite the toxicity issue, in that reduction of mercury far outweighs the toxicity of nZVI (Chapman, Moore & Campbell, 2020). Therefore, in order to have an environmentally friendly nZVI situation, one needs to balance the usability of nanoscale zero-valent iron with its toxic influences.
Challenges and future prospects in using zero-valent iron nanoparticles technology for mercury removal
While there are numerous benefits of using nZVI for heavy metal remediation, it also has some drawbacks. In an aqueous solution, zero-valent iron nanoparticles were found to be oxidised by oxygen and water, resulting in delay or complete ceasure of reduction progression, thereby hampering its usefulness. The ability of zero-valent iron nanoparticles to accumulate, resulting in a reduced surface area and mobility, has been well documented. Furthermore, it is also difficult to separate nZVI and its corrosive products from wastewater. However, different strategic methods of modification were being attained, which may include chemical surface amendment or other metals (Pt, Pd, Cu, Ni, etc.) doping on nZVI, as was discussed in sub-section 3.2. In the attempt to address these challenges, one example of modification strategy is the synthesis of a novel, modified nano zero-valent iron that has a higher maximum removal ability towards Cr (VI) using sodium dodecyl sulfate (SDS), a surfactant of anionic form with outstanding capabilities to migrate and disperse. This method can be a promising adsorbent with improvement in adsorption capability and reduced accumulation. A projection of its usage can be extended to other toxic heavy metals like Hg(II) (Yang et al., 2019).
Because of its incredible surface resistance and functioning internal magnetic interactions, nano zerovalent iron is prone to forming sequences-like or larger (micron size) clusters or collections, lowering its movement and responsiveness. Nano zerovalent iron has a high-level potential for reduction, but due to quick oxidation, a thin layer of iron oxides/oxyhydroxides is often deposited on the surface, resulting in reduced reactivity. Moreover, when trying to make it usable in an actual situation, the ultrafine dimension of nanoparticulate zerovalent iron makes it difficult to use. In order to stop this from happening, several surface-modification approaches like doping nano zero-valent iron with metal, coating the nZVI surface, bimetallic nanoparticle admixing with nZVI, support adhesion of nZVI, emulsification of nZVI, modification by magnetization, as well as several other approaches have been utilized to enhance zero-valent iron nanoparticle’s properties (Ken & Sinha, 2020).
The modifier sheet’s surface layer on the nanoparticle’s surface may generally be used to change the surface. As stated earlier, the surface area, magnetic property, its easy method of modification and affordability, among others, are some of the advantages of its usage. That said, the nano zero-valent iron’s ability to remove mercury from wastewater, groundwater and soil still needs to be improved if it is to attain maximum mercury removal. On that note, the surface area is one important factor that can facilitate the needed improvement in the removal processes. This can be achieved through enhancing the different synthetic processes used.
Conclusions
The methods of surface-modification of the nanoscale zero-valent iron have been found promising for the indemnification of toxic compounds from the environs owing to their inexpensiveness, harmlessness, simple and ecologically responsive character. The major concerns associated with zero-valent iron nanoparticles were stability, agglomeration, and transportation. These problems have been resolved to some extent, but not fully; hence, further investigations are needed.
With the speedy improvement and wide-ranging functions of mercury and its complexes, its environmental discharge is unavoidable as well as toxic. Consequently, the reduction/adsorption of the speedy nature of mercury ions has emerged as specifically imperative as well as vital because of its elevated activity coupled with environmental poisoning. By and large, in view of the fact that the reduction/adsorption performance has powerful effects such as poisoning, gathering, sticking together as well as relocation of different weighty ionic metals in the environs, particularly reserves found in clay, ZVI nanoparticles, as an essentially environmental and compassionate-reducing element, have revealed enormous abilities for the elimination of mercury and other contaminants as well as being an in-situ remedy for the environment. It is as vital as it is significant to study the reduction in mercury ions on ZVI nanoparticles from aqueous mixtures coupled with studying the adsorption manners. It would also be useful to be aware of the relocation guides together with ionic metals’ communication mechanism in an environmental medium, particularly with regard to groundwater, reserves (minerals), clay, and wastewater.
Nowadays, many scientists have established that the mechanism of adsorption for substantial ionic metals on ZVI nanoparticles, as far as normal environs are concerned, is to be ascribed to a monolayer molecule sorption and chemical reaction by batch adsorption experimentation. The results of batch experiments have also confirmed that the most favorable adsorption pH values procedure is 4.0–7.0 on the majority of ZVI nanoparticles. This also matched a particular range in the marine environment, i.e pH of 5.0–9.0, as well as a majority of nano ZVI complexes with outstanding characteristics demonstrating enormous adsorption ability and elimination behavior (Zou et al., 2016). These results offered a crucial breakthrough as regards the adsorption and fate of substantial metals ions on ZVI nanoparticles in a normal environ as well as allowing to some extent for evaluating the environmental effect. Simultaneously, ZVI nanoparticle adsorbent’s effectiveness and reduction abilities were anticipated, aimed at substantial metals ions and utilized for environs’ remedy, which might supply an easy process for the effective removal of mercury and other toxic compounds in the environment from aqueous mixtures. To be precise, ZVI nanoparticles can be a reliable substance in eliminating a substantial amount of mercury ions effectively from aqueous mixtures by utilizing a simple and chemically fast reaction, in addition to an adsorption mechanism. Although worldwide approval of the ZVI nanoparticle as a remedial substance is yet to happen, more knowledge of the environmental manners, methods of environmental remedy, and the influencing factor of mercury ions adsorbed by ZVI nanoparticles is also yet to be established. Future studies will do well to set up a comprehensive figure of proof on the communication procedure of ZVI nanoparticles in a normal setting which might well prove the in-situ remedial effectiveness of the ZVI nanoparticle administered environmentally as well as its reductive effect on mercury poisoning, otherwise another widespread toxic compound.