Antimicrobial and antioxidant potential from Piper marginatum roots
- Published
- Accepted
- Received
- Academic Editor
- Antonio Crotti
- Subject Areas
- Bioorganic Chemistry, Natural Products
- Keywords
- Piperaceae, Piper marginatum, Antimicrobial, Antioxidant, Phenolic content, Alkylamide, Antimicrobial
- Copyright
- © 2023 Soares da Silva et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ Organic Chemistry) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Antimicrobial and antioxidant potential from Piper marginatum roots. PeerJ Organic Chemistry 5:e8 https://doi.org/10.7717/peerj-ochem.8
Abstract
This is the first report of the antimicrobial and antioxidant potential of extract from Piper marginatum roots. The extract showed highest antioxidant activity with an EC50 of 47.3 ± 0.80 µg/mL and a total phenolic content of 42.7 ± 1.10 mg GAE/g. The extract exhibited strong antimicrobial activity with minimum inhibitory concentrations of 250, 250 and 125 µg/mL for the bacteria Bacillus subtilis, Staphylococcus aureus and fungus Mycobacterium smegmatis. Antimicrobial activity was attributed to (E,E)-N-Isobutyl -2,4-octadienamide and (E,E)-N-Isobutyl-2,4-decadienamide amides isolated as major compounds of the roots. Structural elucidation of the two amides was determined based on the interpretation of their IR, UV, MS, 1H and 13C NMR spectra. The results contribute significantly to the development of a herbal remedies based on P. marginatum roots.
Introduction
Natural products have been considered an excellent source of new molecules as a prototype in the discovery of new drugs (Silva et al., 2021). In the period of 30 years (1981 to 2014), 43.5% of the drugs approved worldwide for the treatment of infections caused by bacteria, fungi, parasites and viruses were obtained from natural products (Cragg & Newman, 2013). Taxol is one of the remarkable natural anticancer drugs, firstly extracted from the Taxus brevifolia plant in 1971 and has been useful in the management of many cancers (Teibo et al., 2023). Artemisinin is another example of a natural source drug; a sesquiterpene extracted from Artemisia annua and widely used in Chinese medicine for the treatment of malaria (Weathers, 2023).
As part of our systematic chemical and biological study with plant species of the Piperaceae family (Da Silva et al., 2018; Rocha et al., 2016; Barbosa et al., 2012), the present work was directed to evaluate the antimicrobial and antioxidant activity as well as the structural determination of the specialized metabolites of extracts from Piper marginatum roots. P. marginatum, popularly known as Pimenta do Mato, Malvaísco or Capeba Cheirosa, is a plant used in folk medicine to relieve stomach pains, as a diuretic, for snake bites and as a carminative (Brú & Guzman, 2016). It has exhibited diverse activities including cytotoxic, larvicidal, antimicrobial, insecticidal, antioxidant, repellent, anti-alimentary and phytotoxic (Macêdo et al., 2020; Brú & Guzman, 2016). Previous chemical studies have revealed that the P. marginatum leaves accumulate prenylated benzoic acids, amides and flavonoids while essential oils were rich in phenylpropanoids (De Oliveira, Silva & Ramos, 2022; Santos Ayres et al., 2022). Previous studies revealed that dichloromethane extract and essential oil from P. marginatum leaves rich in phenylpropanoids exhibited antimicrobial activity against pathogens of clinical importance (Bezerra & Ramos, 2021). Despite wide use of P. marginatum in folk medicine and its variety of biological activities; phytochemical and biological studies with extracts from the roots of P. marginatum are still rare (Takeara et al., 2017; Bay-Hurtado et al., 2016; Sequeda-Castañeda et al., 2015).
Materials & Methods
Chemicals
Folin-Ciocalteu’s phenol reagent, potassium persulfate and 6- hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox) were obtained from Sigma-Aldrich (Waltham, MA, USA), and 2,2- azinobis 3-ethylbenzothiozoline-6-sulfonic acid (ABTS) was supplied by Fluka Chemie (Buchs, Switzerland).
Plant
The roots of P. marginatum were collected in an Atlantic forest fragment located on the Campus of the Federal Rural University of Pernambuco (UFRPE), Recife, Pernambuco, Brazil (8°00′53.4″S 34°57′05.5″W). The botanical material was identified by Prof. Dr. Margarethe Ferreira de Sales from the Department of Biology at UFRPE. A specimen of the collected botanical material was deposited at the Vasconcelos Sobrinho Herbarium of the Department of Biology at UFRPE under number 45870.
Extraction and compounds isolation
P. marginatum roots (500 g) were dried in a circulating air oven for a period of 48 h at 50 °C and the dried material was milled to a fine powder in a Macsalab mill (400 g). The dried root was extracted by maceration with n-butanol (3 × 400 mL) at room temperature for 48 h and concentrated in a vacuum to yield crude extract (8 g). Part of the extract (4.0 g) was subjected to fractionation on a Sephadex LH-20 (Amersham Biosciences, Amersham, UK) using methanol as eluent, yielding 84 fractions. Fractions 23 to 31 were pooled (350 mg) and further fractionated with silica gel (Merck 230-400 mesh) using hexane with increasing amounts of ethyl acetate (EtOAc) as the eluent, yielding 40 fractions with isolation of compounds 1 (20.4 mg) and 2 (18.6 mg).
HPLC analyses
HPLC analyses of extracts and the pure compound were performed using a Shimadzu LC10 instrument (Tokyo, Japan) with an SPD-M20A diode array detector using a C18 column (250 mm, 4.6 mm, 5 µM, (Supelco, Bellefonte, PA, USA) eluted in a gradient mode starting with CH3OH:H2O (3:7) for 10 min, raising to 100% of CH3OH (HPLC grade, Merck-Brazil, Millipore, Burlington, MA, USA) in 40 min and flow rate of 1.0 mL/min. The injection volume was 20 µL of solution 1 mg/mL in methanol.
Structure
1H and 13C NMR spectra of 1 and 2 compounds were acquired in CDCl3 on Bruker DPX-300 (Bruker, BioSpin GmbH Silberstreifen 476287 Rheinstetten Germany; 300 MHz and 75 MHz, respectively) spectrometer with pulsed field gradient and signals referenced to the residual solvent signals (CDCl3, at δ H 7.26 and δ C 77.0 ppm, 99% purity Aldrich, St. Louis, MO, USA). GC-MS analyses (60−260 °C at 3 °C/min. heating rate) were carried out in a Varian 431-GC coupled to a Varian 220-MS instrument using (Palo Alto, CA, USA) fused-silica capillary column (30 m × 0.25 mm i.d. × 0.25 µm) coated with DB-5. MS spectra were obtained using electron impact at 70 eV with a scan interval of 0.5 s and fragments from 40 to 550 Da. The injection volume was 1.0 µL of solution 1 mg/mL in methanol. IR spectra were obtained on a Perkin Elmer Nicolet 1750 using KBr disk (Palo Alto, CA, USA). The analyses UV-Vis were carried out Agilent 8453 spectrophotometer (Santa Clara, CA, USA), in the interval from 190 to 600 nm, using 10-mm quartz cuvettes and solution (0.5 mg/mL) in methanol.
Total phenolic content
The total phenolic content of the samples was determined using the Folin-Ciocalteu reagent (Sigma-Aldrich, St. Louis, MO, USA) according to the method previously reported with slight modifications of gallic acid as a standard phenolic compound (Slinkard & Singleton, 1977). Appropriate amounts of each sample (500 µL; 50 µg/mL) were diluted in a volumetric flask with distilled water (3 mL). The Folin-Ciocalteu reagent (100 µL) was added and the contents of the flask were thoroughly mixed. After 3 min, Na2CO3 (15%, 300 µL) was added and the mixture was completed with distilled water (5 mL) and allowed to stand for 2 h in an ultrasonic bath. The absorbance was measured at 760 nm in a spectrophotometer. The total amount of phenolic compounds was determined in micrograms of gallic acid equivalents, using the equation obtained from the standard gallic acid graph.
ABTS•+ radical scavenging assay
The method previously reported with slight modifications was adopted for ABTS (2,2′-azino-bis-(3-ethylbenzothiazoline-6-sulfonate, 98% purity) assay (Re et al., 1999). ABTS•+ was generated by reacting ABTS solution (7 mM) with potassium persulphate (140 mM, final concentration) for 16 h in the dark at room temperature. Then, the ABTS•+ solution was diluted with ethanol to obtain absorbance of 0.70 (± 0.02) at 734 nm and further equilibrated at 30 °C. The samples were diluted in ethanol at a concentration of 1 mg/mL, and the ABTS•+ solution was added to both samples to obtain concentrations of 1.0 to 100.0 µg/mL. The absorbance of the reaction mixture was measured at 734 nm after reaction at room temperature for 10 min. Trolox (97% purity) was used as positive control. The capability of scavenging the ABTS•+ radical was calculated using the following equation: ABTS•+ scavenging effect (%) = [(A0 − A1 / A0) ×100].
Where A0 is the initial concentration of ABTS•+ and A1 is the absorbance of the remaining concentration of ABTS•+ in the presence of the sample.
In vitro antimicrobial activity
The antimicrobial potential of extract and compounds was evaluated against the gram-positive bacteria Staphylococcus aureus, S. aureus MRS, Bacillus subtilis and the gram-negative bacteria Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa as well as the fungi Candida albicans, Mycobacterium smegmatis and Malassezia furfur. The bacteria and fungi came from the collection of microorganisms from the Antibiotics Department of the Federal University of Pernambuco. The suspension of microorganisms was standardized by the turbidity equivalent to a 0.5 tube on the McFarland scale in distilled water, corresponding to a concentration of approximately 108 CFU/ml for bacteria and 107 CFU/ml for fungi (Ramos et al., 2022; Bezerra & Ramos, 2021).
Determination of the minimum inhibitory concentration (MIC)
MIC was performed using the microdilution technique in 96-well multiplates. The culture media used were Sabourand Agar (for fungus) and Muelle-Hinton Agar (for bacteria). Metronidazole (2.5 µg/ml) and Fluconazole (2.5 µg/ml) were used as a positive control, while ethyl alcohol was used as a negative control. Analyses were performed in triplicate and the microplates were cultured at 37 °C for 18–24 h for bacteria and 30 °C for 48–72 h for the fungus. After the culture period, the microplates were developed with the addition of 10 µL of 0.01% resazurin solution and incubated for 3 h. The MIC was defined as the lowest concentration of the sample that inhibited the growth of the microorganisms (Ramos et al., 2022; Bezerra & Ramos, 2021).
Result & Discussion
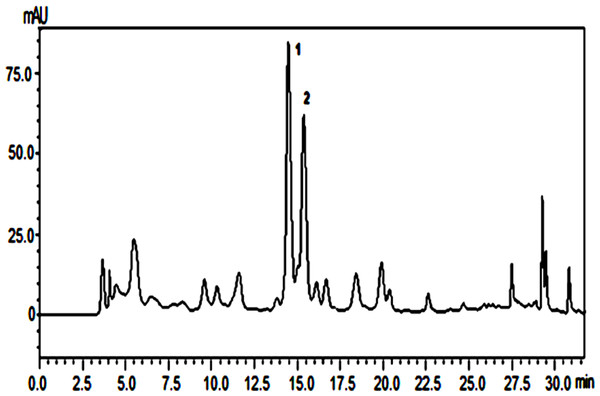
The chemical profile obtained by HPLC of the butanolic extract from P. marginatum roots showed two major peaks (Fig. 1).
Figure 1: Chemical profile of butanolic extract from P. marginatum roots.
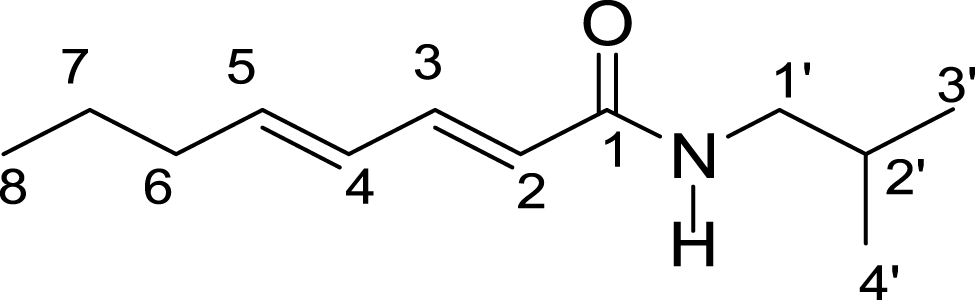
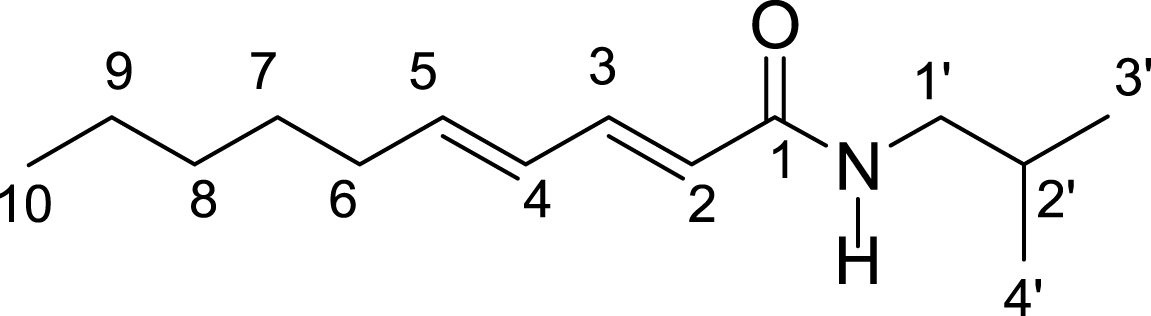
The extracts were submitted to purification steps by chromatographic methods enabling isolation of two compounds 1 and 2. The UV, IR, 13C and 1H NMR spectra for compounds 1 and 2 were identical, indicating that the chemical structures of these compounds are similar. 13C and 1H NMR spectra of compound 1 showed a set of signals characteristic of alkylamides (Table 1). Mass spectrum for compound 1 showed a molecular ion peak of m/z = 195 Da, and the mass spectrum of compound 2 showed molecular ion peak of m/z = 223 according to the molecular formulas C12H21NO and C14H25NO, respectively. Based on interpretation of IR, MS, 13C and 1H NMR spectral data, compounds 1 and 2 were determined to be (E,E)-N-Isobutyl-2,4-octadienamide and (E,E)-N-Isobutyl-2,4-decadienamide amides, respectively.
| H/C | (E,E)-N-Isobutyl-2,4-octadienamide (1) | (E,E)-N-Isobutyl-2,4-decadienamide (2) | ||
|---|---|---|---|---|
| H/C |
|
|
||
| δH | δC | δH | δC | |
| 1 | – | 166.77 | – | 166.40 |
| 2 | 5.75 (d, 1H, J = 14.9 Hz) | 143.30 | 5.70 (d,1H, J = 15.2 Hz) | 143.31 |
| 3 | 6.09 (m, 2H) | 141.68 | 6.12 (m. 2H) | 141.38 |
| 4 | 6.09 (m, 2H) | 128.77 | 6.12 (m, 2H) | 128.19 |
| 5 | 7.18 (dd, 1H, J = 14.9 and 9.6 Hz) | 122.17 | 7.20 (dd, 1H, J = 15.2 and 9.9 Hz) | 121.67 |
| 6 | 2.12 (q, 2H, J = 7.0 Hz) | 35.38 | 2.14 (q, 2H, J = 5.5 Hz) | 32.93 |
| 7 | 1.44 (m, 2H) | 29.05 | 1.79 (m, 2H) | 31.38 |
| 8 | 0.91 (m, 3H) | 14.08 | 1.29 (m, 2H) | 28.64 |
| 9 | – | – | 1.40 (m, 2H) | 28.49 |
| 10 | – | – | 0.91 (t, 3H) | 14.02 |
| 1′ | 3.16 (d, 2H, J = 6.7) | 47.32 | 3.16 (d, 2H, J = 6.7 Hz) | 46.93 |
| 2′ | 1.81 (m, 1H) | 22.43 | 1.80 (m, 2H) | 22.48 |
| 3′ | 0.90 (d, 6H, J = 7.0) | 20.54 | 0.90 (d, 6H, J = 6.7 Hz) | 20.13 |
| 4′ | 0.90 (d, 6H, J = 7.0) | 20.54 | 0.90(d, 6H, J = 6.7 Hz) | 20.13 |
| N-H | 5.51(s, 1H) | – | 5.45 (s,1H) | – |
The (E,E)-N-Isobutyl-2,4-octadienamide and (E,E)-N-Isobutyl-2,4-decadienamide amides previously isolated from Piper sarmentosum aerial parts, P. nigrum seeds and Cissampelos glaberrima roots showed insecticidal activity (Siddiqui et al., 2005; Stöhr, Xiao & Bauer, 1999; Rosario, Da Silva & Parente, 1996). This is the first report of E,E)-N-Isobutyl-2,4-decadienamideamide in P. marginatum tissues. The (E,E)-N-Isobutyl-2,4-octadienamide has been previously of aqueous ethanolic extract from P. marginatum roots (De Oliveira Santos & De Oliveira Chaves, 1999).
Antimicrobial activity
The crude extract from P. marginatum roots was tested against nine microorganisms and showed an inhibition zone for two bacteria and one fungus, indicating a selective antimicrobial activity for these microorganisms (Table 2).
| Microorganisms | Zone of inhibition (mm) | MIC (µg/ml) | |||
|---|---|---|---|---|---|
| Extract | Extract | Compound 1 | Compound 2 | ||
| B. subtilis | 11 | 250 | 250 | 250 | |
| S. aureus | 22 | 250 | 250 | 250 | |
| S. aureus MRS | 0 | – | – | – | |
| K. pneumoniae | 0 | – | – | – | |
| E. coli | 0 | – | – | – | |
| P. aeruginosa | 0 | – | – | – | |
| M. furfur | 0 | – | – | – | |
| C. albincas | 0 | – | – | – | |
| M. smegmatis | 15 | 125 | 125 | 125 | |
The minimum inhibitory concentrations (MIC) of the extract for the bacteria B. subtilis, S. aureus and fungus M. smegmatis were determined with values of 250, 250 and 125 µg/mL, respectively (Table 2). MIC values ≤ 500 µg/ml are considered strong inhibitors; MIC values from 600 to 1,500 µg/ml are considered moderate inhibitors; values 1,500 µg/ml are considered weak inhibitors (Aligiannis et al., 2001). The (E,E)-N-Isobutyl-2,4-octadienamide and (E,E)-N-Isobutyl-2,4-decadienamide amides showed strong antimicrobial activity against B. subtilis, S. aureus and M. smegmatis with value of 250 µg/mL for bacteria and 125 µg/mL for fungus, indicating that the amides are responsible for activity of butanolic extract from P. marginatum roots. A previous study revealed that essential oil from leaves of P. marginatum rich in phenylpropanoids showed antimicrobial activity against bacteria B. subtilis, S. aureus and fungus M. smegmatis with MIC values of 250, 2,500, 1,250 µg/mL, respectively (Ramos et al., 2022; De Oliveira, Silva & Ramos, 2022). The leaf extract showed activity against the bacteria S. aureus with a MIC of 1,250 µg/mL (Bezerra & Ramos, 2021).
Antioxidant activity and total phenolic content
The results obtained in the determination of total phenolics were expressed in milligrams equivalent of gallic acid per gram of extract (mg GAE/g, Table 3).
| Samples | ABTS EC50 µg/mL | Total phenolic amg GAE/g |
|---|---|---|
| Extract | 47.3 ± 0,80 | 42.7 ± 1,10 |
| (E,E)-N-Isobutyl-2,4-octadienamide | 383.0 ± 5.19 | – |
| (E,E)-N-Isobutyl-2,4-decadienamide | 340.4 ± 4.81 | – |
| Trolox | 2.8 ± 0,00 | – |
Notes:
The crude root extract exhibited a total phenolic content of 42.7 ± 1.10 mg GAE/g, indicating that in addition to amides, the root extract also accumulates phenolic compounds in moderate amounts. The butanolic extract from P. marginatum roots showed the highest activity with an EC50 = 47.3 ± 0.80 µg/mL. ABTS⋅+ radical scavenging activity of roots was associated with the phenolic content, considering that EC50 = for the 383.0 ± 5.19 and 340.4 ± 4.81 µg/mL, respectively, considering that values approaching 500 µg/mL do not exhibit good scavenging capacity. To our knowledge, this is the first report of the antioxidant activity for the extract and their major constituents from the roots of P. marginatum. Previous studies with essential oil of P. marginatum showed significant antioxidant activity with a DPPH IC50 value between 1,200 and 1,500 µg/ml while the control ascorbic acid showed a DPPH IC50 value of 1,000 µg/ml (Brú & Guzman, 2016). Essential oil from the roots of P. marginatum exhibited antioxidant activity with a DPPH IC50 value of 75,300 µg /L while the Ginkgo biloba used as reference exhibited a DPPH IC50 value of 46.9 mg/L (Bay-Hurtado et al., 2016). Piper umbellata has been reported to be a potent antioxidant attributed to its major constituent, 4-nerolidylcatechol (Cordeiro et al., 2013). The total phenolic concentration of the extract from the leaves of P. umbellata was of 148 mg GAE/g while antioxidant activity exhibited an EC50 of 120.1 µg/mL based on ABTS (Ramos et al., 2012).
Conclusion
This is the first report of the antimicrobial and antioxidant potential of extract from P. marginatum roots. The results revealed that the plant exhibits a selective antimicrobial activity against the bacteria B. subtilis, S. aureus and fungus M. smegmatis attributed to (E,E)-N-Isobutyl -2,4-octadienamide and (E,E)-N-Isobutyl-2,4-decadienamide amides. The extract also exhibited antioxidant activity which was attributed to the phenolic content present in the plant. The results contribute significantly to the chemical and biological knowledge of P. marginatum, a plant widely used in folk medicine.