Effects of ferulic acid esterase-producing Lactobacillus fermentum and cellulase additives on the fermentation quality and microbial community of alfalfa silage
- Published
- Accepted
- Received
- Academic Editor
- Robert Winkler
- Subject Areas
- Agricultural Science, Biotechnology, Microbiology, Plant Science
- Keywords
- Lactobacillus fermentum, Ferulic acid esterase, Cellulase, Alfalfa, Silage
- Copyright
- © 2019 Su et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Effects of ferulic acid esterase-producing Lactobacillus fermentum and cellulase additives on the fermentation quality and microbial community of alfalfa silage. PeerJ 7:e7712 https://doi.org/10.7717/peerj.7712
Abstract
Background
Alfalfa (Medicago sativa) is an important forage material widely used for animal feed production. Ensiling is an effective method for preserving alfalfa, but it has shown some limitations in the production of high-quality alfalfa silage due to its low water soluble carbohydrates (WSC) content and high buffering capacity. Lactic acid bacteria (LAB) and cellulase are often used as silage additives to promote the ensiling process and enhance fermentation quality.
Methods
Experiments were conducted to investigate the effects of ferulic acid esterase (FAE)-producing Lactobacillus fermentum 17SD-2 (LF) and cellulase (CE) on the fermentation quality and microbial community of alfalfa silage. After 60 days of ensiling, analysis of fermentation quality and bacterial diversity in alfalfa silages were conducted using high-performance liquid chromatography and high-throughput sequencing methods.
Results
Alfalfa was ensiled with additives (LF, CE, and LF+CE) or without additives for 60 days. All additives increased lactic acid and decreased pH values and ammonia-N contents compared to control. Among all treatments, the combined addition of LF and CE showed lowest pH (4.66) and ammonia-N (NH3-N, 0.57% DM) content, highest contents of lactic acid (LA, 10.51% DM), dry matter (DM, 22.54%) and crude protein (CP, 24.60% DM). Combined addition of LF and CE performed better in reducing neutral detergent fiber (NDF, 29.76% DM) and acid detergent fiber (ADF, 22.86% DM) contents than the addition of LF (33.71, 27.39% DM) or CE (32.07, 25.45% DM) alone. Moreover, the microbial analysis indicated that LF+CE treatments increased the abundance of desirable Lactobacillus and inhibited the growth of detrimental Enterobacter and Clostridia in alfalfa silage.
Discussion
Combined addition of FAE-producing LF and CE is more effective than treatments of LF or CE alone in improving fermentation quality and nutrition values of alfalfa silage. This is likely due to a synergistic effect of CE and FAE produced by LF on plant cell wall degradation, indicating that these additives promote each other to improve fiber degradation and silage fermentation. In conclusion, combined addition of FAE-producing LF and CE could be a feasible way to improve alfalfa silage quality.
Introduction
Alfalfa is widely used for animal feed in the world due to its high protein content. However, the low concentration of WSC and high buffering capacity in alfalfa makes it difficult to ensile for high silage quality (Silva et al., 2016). In order to enhance fermentation quality, lactic acid bacteria (LAB) and cellulase are often used as silage additives. LAB can accelerate lactic acid fermentation and reduce nutrition loss during the ensiling process (Gulfam et al., 2017). Cellulase can degrade the plant cell wall and improve the ruminal digestibility of silage (Khota et al., 2016).
Ferulic acid ester usually links ferulic acid with polysaccharides in plant cell walls, which results in extended networks and forms a protective layer. Ferulic acid esterase (FAE) can break the ester linkage between the ferulate and polysaccharide chain, and then enhance the accessibility of cellulase and hydrolyze the cell wall (Koseki et al., 2009). Well-characterized FAEs are mainly from fungi (Faulds et al., 2005), but now some researchers are increasingly studying FAE-producing bacteria due to their practical application value (Fritsch et al., 2017).
For silage fermentation, Jin et al. reported that mixed small-grain silage treated with FAE-producing Lactobacillus buchneri had higher in situ NDF digestibility than control. Furthermore, pretreatment with L. plantarum A1 (FAE activity) alone or in combination with exogenous cellulase had a synergistic effect on corn stalk silage fermentation and degradation of lignocellulase (Li et al., 2019), such as producing more available substrates for LAB fermentation. However, the effects of FAE-producing LAB inoculant on silage quality can be highly variable, possibly due to the different forage varieties and performance differences in LAB strains (Lynch, Baah & Beauchemin, 2015; Jin et al., 2017; Li et al., 2019).
The potential of FAE activity LAB to improve silage quality or fiber digestibility of various silages includes barley, ryegrass and corn have been reported (Addah et al., 2012; Nsereko et al., 2008; Kang et al., 2009). But there is little information about the application of FAE activity LAB or its combination with exogenous cellulase on alfalfa silage. We hypothesized that the combination of FAE-producing LF and CE will improve the fermentation quality and nutrition values more effectively in alfalfa silage. In order to verify the hypothesis, experiments were carried out as follows: characterize FAE activity L. fermentum 17SD-2; investigate the effects of L. fermentum 17SD-2 (LF), cellulase (CE), or their combination on the fermentation quality and bacterial community of alfalfa silage.
Materials and Methods
Identification of FAE activity in Lactobacillus fermentum 17SD-2
Lactobacillus fermentum 17SD-2 used in this study was isolated from maize silage without any additives, then maintained in De Man, Rogosa, and Sharpe (MRS) broth containing 20% (v/v) glycerol at −80 °C. Before any experiments, the strain was propagated twice and cultured in MRS broth for 24 h at 37 °C, harvested by centrifugation (10,000 rpm, 2 min), and washed twice with phosphate buffered saline (PBS, pH 7.0). The sediment was resuspended in sterile water and 10 µL of the suspension was transformed to MRS plates with glucose omitted but containing 1g/L ethyl ferulate and incubated for 72 h at 37 °C, and Lactobacillus fermentum 5007 (CGMCC 1.3223) was used as negative control because it was previously confirmed to have no FAE activity. After incubation, we observed whether a clear zone formed around the suspension. If a clear zone appeared on the MRS plates, this indicated that the strain L. fermentum 17SD-2 had FAE activity.
Morphological, physiological, and biochemical characteristics of Lactobacillus fermentum 17SD-2
Gram staining and catalase activity of L. fermentum 17SD-2 were determined after 24 h of incubation on MRS agar or broth. To measure the growth curve of Lactobacillus fermentum 17SD-2, the bacterial cells at a density of 1 ×107 CFUs/ml were seeded in MRS broth. The absorbance of Lactobacillus fermentum 17SD-2 culture at 600 nm were regularly monitored with a microplate reader. To evaluate salt tolerance, 2% culture was inoculated into MRS broth containing 3.0% and 6.5% NaCl, and then cultured at 37 °C for 24 h. To evaluate acid tolerance, 2% culture was inoculated into MRS broth with pH 3.5, 4.0, 4.5, 8.0, 8.5, and 9.0, and then cultured at 37 °C for 24 h. To evaluate temperature tolerance, 2% culture was inoculated into MRS broth, and then cultured at different temperatures (including 15, 20, 25, 30, 35, 40, and 45 °C) for 24 h. Then the absorbance of Lactobacillus fermentum 17SD-2 cultures at 600 nm was measured with a microplate reader. The value of OD600 greater than 0.3 was considered as positive, expressed with “+”. API 50CH contains a high level of carbohydrates and was used to determine carbon source utilization according to the manufacturer instructions.
Alfalfa harvest and silage preparation
Alfalfa at blooming stage was manually harvested from an experimental field with an area of 5 m ×10 m, located at the Wuqing District (117°10′E, 39°10′N), Tianjin, China on June 7th, 2018. At first, the harvested alfalfa was divided into two groups: fresh (sample1, S1) and wilted (sample 2, S2, wilted in the field for 5 h). Alfalfa with various dry matter (DM) contents (S1: 23.82%; S2: 29.63%) was directly chopped into pieces in length of 2–3 cm using a crop chopper.
A commercial CE (10,000 U/g; Macklin Biochemical Co., Ltd, Shanghai, China) and L. fermentum 17SD-2 were used as additives for silage making. L. fermentum 17SD-2 could grow well at low pH conditions and possess high FAE-activity (clear zone with a diameter of 11.3 mm). The inoculant was added at a level of 106 colony forming unit (CFU) per gram of fresh matter (FM). CE was applied at a ratio of 1 g kg−1 of FM (Chen et al., 2017).
The experimental treatments were designed as follows. Each group (sample 1 and sample 2) included 12 piles (500 g per pile), and they were randomly ensiled with four additives treatments: (i) untreated control (CK); (ii) application of commercial cellulase (CE); (iii) application of L. fermentum 17SD-2 (LF); and (iv) combination of L. fermentum 17SD-2 and commercial CE (LF+CE). Then, chopped material was mixed homogenously with the additives and packed manually into 35 cm × 50 cm polyethylene bags which were tightly vacuumed, and triplicates for per treatment were prepared. A total of 24 bags (2 groups × 4 treatments × 3 replicates) were kept at ambient temperature (21–30 °C). After 60 days of ensiling, three bags per treatment were opened to evaluate their fermentation end products, chemical composition, and microbial communities.
Microorganism and fermentation quality analysis
To evaluate microbial counts, 10 g forage (raw materials and silage) was extracted with 90 mL 0.85% sterile physiological saline solution, and serially diluted from 10 −1 to 10−6. In total, 100 µL from an appropriate dilution was spread on agar plates. Lactic acid bacteria were enumerated on MRS agar after incubation at 30 °C for 72 h. Molds and yeasts were counted on potato dextrose agar (Nissui) after incubation at 28 °C for 24 h, and yeasts were distinguished from molds or other bacteria by colony appearance and cell morphology observation (Avila et al., 2009). Plates containing a minimum of 30 and a maximum of 300 colony-forming units were enumerated (Reich & Kung, 2010). To determine the fermentation parameters, the sample (25 g) was mixed with 225 mL sterile water and incubated at 4 °C overnight and then filtered through four layers of cheesecloth (Touno et al., 2014). The filtrate was used to measure pH and the concentrations of ammonia-N and organic acid. The ammonia-N content was determined according to phenol-hypochlorite and ninhydrin colorimetric procedures described in a previous study (Broderick & Kang, 1980). Organic acids were analyzed using high-performance liquid chromatography (HPLC), equipped with a UV detector and set as follows: ICSep COREGEL-87H column, eluent five mmol/L H2SO4 with a running rate of 0.6 mL/min, temperature of column oven 50 °C, injection volume of 10 µL.
Analysis of chemical composition
To determine the DM content, the samples were dried in a forced-air oven at 65 °C for 48 h and then dried at 103 °C to constant weight. The silage DM losses during drying were calculated according to the formula of Porter et al. (1995). Dried sample was grinded to 1.0 mm particle diameter. The WSC was determined using the anthrone method (Murphy, 1958) and the CP was analyzed according to the standard procedure detailed by the Association of Official Analytical Chemists (AOAC, 1990). The content of NDF and ADF were measured according to the method described in a previous study (Van Soest, Robertson & Lewis, 1991).
Bacterial diversity analysis
Samples (20 g) were mixed with 180 mL of sterile 0.85% NaCl solution with vigorous shaking at 120 r/m for 2 h. The mixture was filtered through four layers cheesecloth and the filtrate was centrifuged at 10,000 r/m for 10 min at 4 °C. The deposit was resuspended in one mL of sterile 0.85% NaCl solution and the microbial pellets were obtained by centrifugation at 12, 000 r/m for 10 min at 4 °C. To extract total DNA in raw material and silage samples (30 samples in total), the DNA Easy Power Soil Kit (Qiagen, Hilden, Germany) was used according to the manufacture’s protocols. The PCR reactions were conducted in a 20 µL mixture (10 ng of template DNA, 2 µL of 2.5 mM dNTPs, 0.8 µL of each primer (5 µM), 0.4 µL of FastPfu Polymerase, and 4 µL of 5 ×FastPfu Buffer, 0.2 µL of BSA, 11.8 µl of ddH2O). According to Ni et al. (2017), the 16S rDNA V3-V4 regions were amplified using primers 338F (ACTCCTACGGGAGGCAGCAG) and 806R (GGACTACHVGGGTWTCTAAT). Triplicate PCR reaction for each sample was conducted to minimize PCR deviation, and a mixture of the three PCR products was sequenced.
High-throughput sequencing and bacterial diversity analysis
Amplicons were extracted from 2% agarose gels, purified with a Qiagen Gel Extraction Kit (Qiagen, Hilden, Germany) and sequenced at the Shanghai Majorbio Bio-pharm Technology Co., Ltd using paired-end sequencing (2 ×300 bp) with an Illumina MiSeq platform according to the standard protocols. Raw tags were quality-filtered by Trimmomatic (Version 3.29) and merged by FLASH (Version 1.2.7) with the following criteria: (i) the reads were truncated at any site receiving an average quality score <20 over a 50 bp sliding window; (ii) sequences with overlaps longer than 10 bp were merged according to their overlap with mismatches no more than 2 bp; and (iii) sequences of each sample were separated according to barcodes (exactly matching) and primers (allowing 2 nucleotides mismatching) and reads containing ambiguous bases were removed. Operational taxonomic units (OTUs) were clustered with 97% similarity using UPARSE (version 7.1, http://drive5.com/uparse/). OTUs were used to calculate rarefaction and alpha diversity (Mothur (v.1.30.1).
Statistical analysis
Data shown are means ± standard deviation (SD). All microbial counts data were converted to log10 and the results were described on a fresh weight basis. Two-way analysis of variance was used to evaluate the effects of water contents, additives and their interaction on microbial population, pH values, fermentation characteristic, chemical composition and alpha diversity of bacterial community in alfalfa silage. All the statistical analyses were performed using the general linear model procedure of SAS (version 9.0, 2002; SAS Institute, Cary, NC, USA). The means were compared for significance by Duncan’s multiple range method and the significance was declared at P <0.05.
Results
Chemical and microbial composition of raw materials and the characteristics of Lactobacillus fermentum 17SD-2
The chemical composition and microbial populations of the alfalfa crops before ensiling are shown in Table 1. The DM contents of sample 1 and sample 2 were 23.82% and 29.63% of FM, respectively; their CP contents were 22.63 and 25.34% DM, respectively; and NDF and ADF concentrations were 33.3–38.3% and 25.5–29.7% DM, respectively. In this study, the WSC contents were lower than 1.8% of DM in both samples. Additionally, the numbers of epiphytic LAB, yeast, and mold were similar between sample 1 and sample 2. The count of LAB was lower than 103 CFU/g FM, while yeast and mold counts were around 104 CFU/g FM.
| Item | S1 | S2 | P value |
|---|---|---|---|
| Chemical composition | |||
| DM% | 23.81 ± 1.12 | 29.63 ± 0.53 | 0.0012 |
| CP (%DM) | 22.62 ± 0.35 | 25.34 ± 0.21 | 0.0003 |
| NDF (%DM) | 38.39 ± 0.02 | 33.35 ± 0.28 | 0.00001 |
| ADF (%DM) | 29.73 ± 0.05 | 25.54 ± 0.4 | 0.0001 |
| WSC (%DM) | 1.54 ± 0.12 | 1.8 ± 0.05 | 0.0219 |
| Cultivable microbial population | |||
| Yeast (log10 cfu/g FM) | 4.38 ± 0.03 | 4.08 ± 0.01 | 0.00005 |
| Mold (log10 cfu/g FM) | 4.2 ± 0.01 | 3.9 ± 0.12 | 0.0099 |
| Alpha diversity of bacterial community | |||
| Observed species | 249.67 ± 17.21 | 175 ± 19.29 | 0.0075 |
| Shannon | 1.94 ± 0.46 | 1 ± 0.02 | 0.0244 |
| Simpson | 0.33 ± 0.05 | 0.61 ± 0.02 | 0.0006 |
| ACE | 327.64 ± 61.36 | 262.24 ± 15.8 | 0.1483 |
| Chao 1 | 309.88 ± 28.96 | 241.81 ± 7.24 | 0.0168 |
| Coverage | >0.99 | >0.99 | NA |
Notes:
- FM
-
fresh material
- DM
-
dry matter
- CP
-
crude protein
- NDF
-
neutral detergent fiber
- ADF
-
acid detergent fiber
- WSC
-
water soluble carbohydrates
- LAB
-
lactic acid bacteria
- CFU
-
colony forming units
- NA
-
not applicable
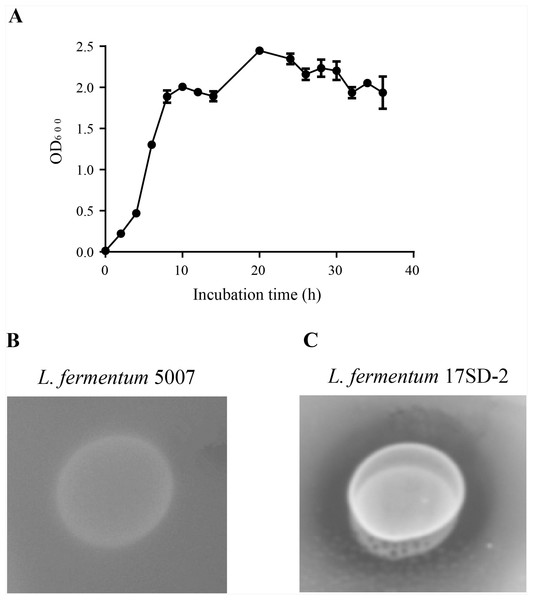
The characteristics of the selected LAB are shown in Table 2. L. fermentum 17SD-2 is Gram-positive, heterofermentation and catalase-negative and was able to grow at a wide range of pH (pH 3.5–9.0), a broad range temperature (15–45 °C), and a salinity of 6.5%. L. fermentum 17SD-2 used the following as carbon sources: ribose, galactose, D-glucose, D-fructose, D-mannose, maltose, lactose, melibiose, sucrose, D-raffinose, gluconate, and 5-keto-gluconate. Furthermore, this strain grew vigorously and possessed high FAE activity (Fig. 1).
| Characteristics | Lactobacillus fermentum 17SD-2 |
|---|---|
| Shape | Rod |
| Gram stain | + |
| Fermentation type | Heterofermentation |
| Catalase | _ |
| Growth at pH | |
| 3.5 | + |
| 4.0 | + |
| 4.5 | + |
| 8.0 | + |
| 8.5 | + |
| 9.0 | + |
| Growth in NaCl | |
| 3.0% | + |
| 6.5% | + |
| Growth at temperature | |
| 15 °C | w |
| 20 °C | w |
| 25 °C | + |
| 30 °C | + |
| 35 °C | + |
| 40 °C | + |
| 45 °C | + |
| Fermentation of carbohydrate | |
| Ribose | + |
| Galactose | + |
| D-glucose | + |
| D-fructose | + |
| D-manose | + |
| Maltose | + |
| Lactose | + |
| Melibiose | + |
| Sucrose | + |
| D-raffinose | + |
| Gluconate | W |
| 5-keto-gluconate | + |
Notes:
- +
-
positive
- -
-
negative
- W
-
weakly positive
Figure 1: Growth curve and plate screening assay showing FAE activity by clear zone around the bacterial suspension.
(A) Growth curve of Lactobacillus fermentum 17SD-2. (B) Lactobacillus fermentum 5007 without FAE activity. (C) Lactobacillus fermentum 17SD-2 with FAE activity.Effects of CE, LF, and LF+CE on the pH values and microbial characteristics of alfalfa silage
The final pH values and microbial populations of sample 1 and sample 2 were similar after ensiling (Table 3). Mold was not detected in all silage, and the significance analysis of main factors revealed that all additives (CE, LF, and CE+LF) significantly increased the LAB (P < 0.001) numbers, but decreased the yeast (P < 0.001) numbers. All treatments had lower (P < 0.001) pH values than those of control, particularly the lowest pH (4.6–4.7) in LF+CE treatments, which was much lower than the addition of CE or LF alone.
| Items | LAB log CFU/g FM | Yeast log CFU/gFM | Mold log CFU/gFM | pH value |
|---|---|---|---|---|
| S1 group | ||||
| CK | 6.9 ± 0.019c | 4.6 ± 0.0071 | ND | 6.8 ± 0.038a |
| CE | 7.3 ± 0.019b | ND | ND | 5 ± 0.026d |
| LF | 7.3 ± 0.015b | ND | ND | 5.4 ± 0.041c |
| LF+CE | 7.4 ± 0.019b | ND | ND | 4.7 ± 0.032e |
| S2 group | ||||
| CK | 6.8 ± 0.024c | 4.5 ± 0.038 | ND | 6.1 ± 0.038b |
| CE | 7.4 ± 0.074b | ND | ND | 5.1 ± 0.018d |
| LF | 7.6 ± 0.046a | ND | ND | 5.3 ± 0.074c |
| LF+CE | 7.4 ± 0.014b | ND | ND | 4.8 ± 0.036e |
| SEM | 0.049 | 0.019 | NA | 0.058 |
| Main effects1 | ||||
| WC | 0.132 | 0.052 | NA | <0.001 |
| A | <0.001 | <0.001 | NA | <0.001 |
| WC ×A | 0.003 | 0.019 | NA | <0.001 |
Notes:
- LAB
-
lactic acid bacteria
- FM
-
fresh matter
- S1
-
Sample1 (fresh)
- S2
-
Sample 2 (wilted in the field for 5 h)
- CK
-
untreated control
- CE
-
application of commercial cellulose
- LF
-
application of L. fermentum 17SD-2
- LF+CE
-
combination of L. fermentum 17SD-2 and commercial CE
- SEM
-
Standard error of mean
- ND
-
not detected
- NA
-
Not applicable
- 1. WC
-
water content
- A
-
Additives
- WC × A
-
the interaction between water content and additives
Effects of CE, LF, and LF+CE on the fermentation quality and chemical composition of alfalfa silage
The fermentation parameters and chemical composition of the alfalfa silage after 60 days of ensiling are shown in Table 4. Lactic acid and acetic acid emerged as the main fermentation end products in all alfalfa silage. All additives remarkably increased the lactic acid content (P < 0.001), in particular, LF+CE had a significant effect on lactic acid and butyric acid content. All treatments had higher lactic acid content and ratio of lactic acid and acetic acid, and LF+CE treatments had the highest lactic acid contents. In this study, all treated silage, especially LF+CE silage, had a lower level of butyric acid content (<1.0% DM), while a large amount of butyric acid (about 2.63% DM) appeared in the control silage of sample 1. In sample 2, no great difference was observed between control and additive treatments (except for LF+CE treatment).
| Items | LA | AA | LA/AA | BA | DM | CP | NH3-N | NDF | ADF | WSC |
|---|---|---|---|---|---|---|---|---|---|---|
| S1 group | ||||||||||
| CK | 2.8 ± 0.13d | 5.3 ± 0.047a | 0.52 ± 0.028d | 2.6 ± 0.11a | 20 ± 0.094e | 18 ± 0.05f | 1.8 ± 0.014a | 38 ± 0.089a | 30 ± 0.095a | 0.58 ± 0.012e |
| CE | 9.8 ± 0.24a | 5.3 ± 0.057a | 1.8 ± 0.026b | 1.2 ± 0.011b | 20 ± 0.078d | 21 ± 0.15e | 0.95 ± 0.026b | 32 ± 0.023d | 25 ± 0.035d | 0.64 ± 0.0088d |
| LF | 6.3 ± 0.36b | 4.7 ± 0.048b | 1.3 ± 0.065c | 0.84 ± 0.06d | 21 ± 0.084d | 21 ± 0.066d | 0.88 ± 0.018c | 34 ± 0.068b | 27 ± 0.071b | 0.52 ± 0.015f |
| LF+CE | 11 ± 0.31a | 4.2 ± 0.039c | 2.5 ± 0.067a | 1 ± 0.068c | 23 ± 0.27c | 25 ± 0.05a | 0.57 ± 0.026de | 28 ± 0.038g | 22 ± 0.076f | 0.98 ± 0.015b |
| S2 group | ||||||||||
| CK | 1.8 ± 0.2e | 2.9 ± 0.023f | 0.62 ± 0.074d | 0.57 ± 0.018e | 27 ± 0.14b | 22 ± 0.043c | 0.53 ± 0.012e | 33 ± 0.084c | 26 ± 0.032c | 1.2 ± 0.018a |
| CE | 5.9 ± 0.38b | 3.6 ± 0.031d | 1.6 ± 0.099b | 0.54 ± 0.012e | 27 ± 0.24b | 24 ± 0.044b | 0.59 ± 0.019d | 29 ± 0.19e | 23 ± 0.09e | 1.2 ± 0.015a |
| LF | 4 ± 0.2c | 3.2 ± 0.058e | 1.2 ± 0.074c | 0.58 ± 0.028e | 27 ± 0.34b | 22 ± 0.077c | 0.47 ± 0.0063f | 34 ± 0.27bc | 26 ± 0.26c | 0.79 ± 0.0088c |
| LF+CE | 6.7 ± 0.21b | 2.9 ± 0.011f | 2.4 ± 0.083a | 1 ± 0.0028c | 30 ± 0.09a | 25 ± 0.061a | 0.38 ± 0.01g | 29 ± 0.16f | 23 ± 0.055e | 1.2 ± 0.0088a |
| SEM | 0.38 | 0.06 | 0.097 | 0.073 | 0.27 | 0.11 | 0.025 | 0.2 | 0.16 | 0.018 |
| Main effects1 | ||||||||||
| WC | <0.001 | <0.001 | 0.103 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| A | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| WC ×A | <0.001 | <0.001 | 0.193 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
Notes:
- LA
-
lactic acid
- AA
-
acetic acid
- LA/AA
-
the ratio of lactic acid and acetic acid
- BA
-
butyric acid
- NH 3 -N
-
ammonia-N
- DM
-
dry matter
- CP
-
crude protein
- NDF
-
neutral detergent fiber
- ADF
-
acid detergent fiber
- WSC
-
water soluble carbohydrates
- LAB
-
lactic acid bacteria
- FM
-
fresh matter
- S1
-
Sample1 (fresh)
- S2
-
Sample 2 (wilted in the field for 5 h)
- CK
-
untreated control
- CE
-
application of commercial cellulose
- LF
-
application of L. fermentum 17SD-2
- LF+CE
-
combination of L. fermentum 17SD-2 and commercial CE
- SEM
-
Standard error of mean
- ND
-
not detected
- NA
-
Not applicable
- 1. WC
-
water content
- A
-
Additives
- WC × A
-
the interaction between water content and additives
Although all additives decreased ammonia-N (P < 0.001) and increased CP (P < 0.001) contents compared to control, the LF+CE treated silage had the highest contents of CP and lowest ammonia-N. Compared with the addition of CE alone, lower NDF and ADF contents were found in LF+CE treatment. Overall, the silage treated with FAE-producing LAB plus cellulase was better than those of the silage treated with either LAB or cellulase alone.
Effects of CE, LF, and LF+CE on microbial communities after 60 days of ensiling
High-throughput sequencing of 16S rRNA gene amplicons was conducted to systematically describe the bacterial communities in the raw material and silage. Alpha diversity of all samples is shown in Table 5. The coverage of all samples was above 0.99, which indicated that the sampling depth adequately captured most of the bacteria. Compared with sample 1, lower Chao and Shannon indexes were observed in sample 2, which was consistent with the results reported by Wang et al. (2018), indicated that wilting (water content) had an important influence on silage microorganism. Lower observed species than pre-ensiled alfalfa crops and that of control appeared in CE alone or with LF treated silage in sample 2. In contrast, in sample 1, observed species in LF and LF+CE treated silages were higher, especially in LF treatment. Although we could not classify the behind reason clearly, probably related to the high number of the epiphytic detrimental microorganisms.
| Items | Observed species | Chao 1 | Shannon | Simpson | ACE | Coverage |
|---|---|---|---|---|---|---|
| S1 group | ||||||
| CK | 85 ± 5.6b | 110 ± 20b,c | 2.4 ± 0.084a | 0.15 ± 0.015c,e | 140 ± 34a,b | 0.99 |
| CE | 57 ± 3.5c,d | 100 ± 26b,c | 2.1 ± 0.014b | 0.17 ± 0.0044c,d | 120 ± 6.7b | 0.99 |
| LF | 130 ± 3.5a | 160 ± 6.8a | 2.4 ± 0.032a | 0.14 ± 0.0064d,e | 180 ± 9.7a | 0.99 |
| LF+CE | 85 ± 1.2b | 150 ± 25a,b | 2 ± 0.074b | 0.2 ± 0.016c | 180 ± 30a | 0.99 |
| S2 group | ||||||
| CK | 80 ± 3.8b | 100 ± 3.4b,c | 2.5 ± 0.084a | 0.12 ± 0.01e | 140 ± 7.4a,b | 0.99 |
| CE | 55 ± 3c,d | 75 ± 11c | 2 ± 0.037b | 0.18 ± 0.0063c,d | 83 ± 19b | 0.99 |
| LF | 66 ± 3.5c | 100 ± 6.5b,c | 1.2 ± 0.076c | 0.51 ± 0.031b | 130 ± 4.3a,b | 0.99 |
| LF+CE | 53 ± 3.8d | 70 ± 10c | 1.1 ± 0.036c | 0.58 ± 0.015a | 85 ± 2.5b | 0.99 |
| SEM | 5.2 | 22 | 0.085 | 0.021 | 26 | NA |
| Main effects1 | ||||||
| WC | <0.001 | 0.001 | <0.001 | <0.001 | 0.003 | NA |
| A | <0.001 | 0.077 | <0.001 | <0.001 | 0.054 | NA |
| WC ×A | <0.001 | 0.237 | <0.001 | <0.001 | 0.093 | NA |
Notes:
- S1
-
Sample1 (fresh)
- S2
-
Sample 2 (wilted in the field for 5 h)
- CK
-
untreated control
- CE
-
application of commercial cellulose
- LF
-
application of L. fermentum 17SD-2
- LF+CE
-
combination of L. fermentum 17SD-2 and commercial CE
- SEM
-
Standard error of mean
- ND
-
not detected
- NA
-
Not applicable
- 1. WC
-
water content
- A
-
Additives
- WC × A
-
the interaction between water content and additives
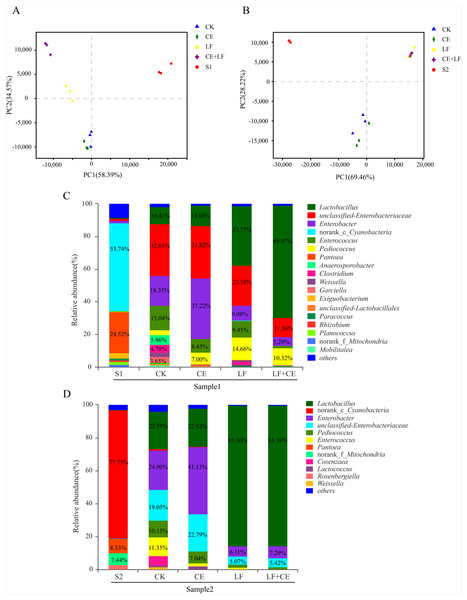
The dynamic variance of microbial population with different treatments can be demonstrated by principle co-ordinates analysis (PCoA). As shown in Fig. 2A (sample 1), component 1 and component 2 could explain 58.07% and 28.51% of the total variance, respectively; Similarity, in Fig. 2B (sample 1), component 1 and component 2 could explain 50.94% and 41%, respectively. Basically, Raw materials S1 and S2 could be well separated from all silage samples, indicating that ensiling was the main factor affecting anaerobic fermentation. Among silage samples, the distance between CK and CE treatments was near, similar phenomenon was observed between LF and LF+CE treatments, indicated that the addition of L. fermentum 17SD-2 had a significant effect on bacterial community in alfalfa silage. In addition, the variation in microbial community might be one critical factor leading to difference in silage quality.
Figure 2: PCoA analysis and relative abundance of bacterial community at the genus level before and after ensiling.
(A) PCoA analysis in sample 1; (B) PCoA analysis in sample 2; (C) Relative abundances of bacterial composition at genus level in sample 1; (D) Relative abundances of bacterial composition at genus level in sample 2. S1, pre-ensiled sample 1; S2, pre-ensiled sample 2; CK, control (without any additives); CE, cellulase; LF, Lactobacillus fermentum 17SD-2; LF+CE, combination of Lactobacillus fermentum 17SD-2 and cellulase.The bacterial communities at the genus level of all alfalfa silage before and after 60 days of ensiling are shown in Figs. 2C and 2D. Before ensiling, in both raw materials (S1 and S2), Cyanobacteria and Pantoea were the most abundant genera, above 53.86% and 8.33% of the entire community, respectively. However, after fermentation, the high abundance of Cyanobacteria and Pantoea significantly decreased or even disappeared. Enterobacter and Lactobacillus gradually became the most prevalent genera. Although Enterobacter was detected in all silage, their relative abundance decreased from 49.98% to 17.01% with the addition of LF and CE, furthermore, LF alone or with CE treated silage showed much lower Enterobacter abundance than control and CE alone treatment. In addition, a similar phenomenon was observed in sample 2.
Lactobacillus, a desirable genus, was present in all silage and Lactobacillus was highly abundant in LF or LF+CE-treated silage, especially in sample 2, where this genus accounted for 85.36% and 85.50% of the total community, respectively. Many other LAB genera, i.e., Pediococcus, Enterococcus, Weissella, and Lactococcus, were also detected after ensiling. For example, in sample 2 silage, the proportions of Pediococcus and Enterococcus ranged from 10.15% to 1.26% and 11.35% to <0.1%, respectively. Weissella and Lactococcus were mainly detected in control and CE treatment. Additionally, relatively high proportions of Anaerosporobacter (5.96%), Clostridium (4.78%), and Garciella (3.65%) were observed in the control of sample 1.
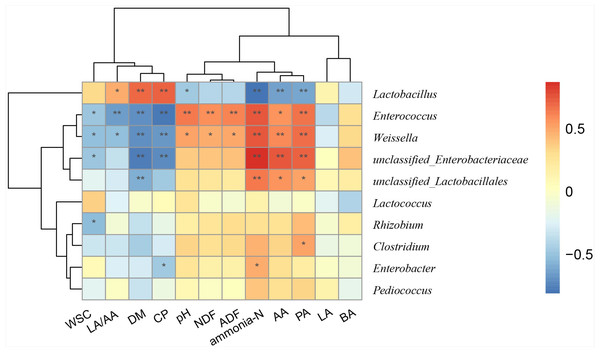
Relationship between main genera and silage quality
To better understand the relationships between bacterial compositions and silage properties, a Spearman correlation heatmap was constructed at the genus level (Fig. 3). Spearman correlation analysis illustrated that Lactobacillus was positively correlated with LA/AA (r = 0.47), DM (r = 0.69), and CP (r = 0.71) contents, while it was negatively correlated with concentration of ammonia-N (r = − 0.81) and pH (r = − 0.47). Enterococcus and Weissella were negatively correlated with LA/AA (r = − 0.65 and −0.51) and positively with pH (r = 0.64 and 0.51) and ammonia-N (r = 0.75 and 0.75). Enterobacter was positively correlated with the concentration of ammonia-N (r = 0.47), but was negatively correlated with CP (r = − 0.47) content.
Figure 3: Spearman correlation heatmap between the main genera and silage quality.
R was presented in different colors; the right side of the legend is the color range of different R values. The value of P ≤ 0.05 is marked with “*”, P ≤ 0.01 is marked with “**”.Discussion
Although ensiling is considered an effective method for preserving high moisture forage, it has shown some limitations in the production of high-quality alfalfa silage due to its low WSC content and high buffering capacity. During the ensiling process, the epiphytic LAB naturally present on forage crops are responsible for silage fermentation and also influence silage quality, which mainly transforms WSC into organic acids, primarily lactic acid, to inhibit the activity of undesirable bacteria. However, in the present study, low WSC contents (below 1.80% DM), a low number of epiphytic LAB (<103 CFU/g of FM) and high buffering capacity may lead to unsatisfactory preservation. Therefore, the ensiling process should be regulated by additions such as cellulase and LAB. In the present study, the selected Lactobacillus fermentum 17SD-2 strain grew well at low pH and it could grow at road temperature. Additionally, this strain grows vigorously and has high FAE activity.
FAE, an important auxiliary enzyme, plays an essential role in plant cell wall hydrolysis by breaking the ester-linked bond between cell wall phenolic acids and polysaccharides, which makes the plant cell wall more accessible to enzyme attack (Faulds, Perez-Boada & Martinez, 2011). Recently, researchers have increasingly reported that most commercial enzyme additives are already mixtures of several fibrolytic enzymes and the effect of these additives on silage chemical composition is actually an integrated effect of synergistic interactions (Li et al., 2018; Li et al., 2014).
As expected, in the current study, compared with addition of CE alone, lower NDF and ADF contents were found in FAE-producing L. fermentum 17SD-2 and cellulase (LF+CE) treatment, and a similar result was found in mixed small-grain silage (Jin et al., 2017). These results indicated that the combination of LF and CE might have a synergistic effect, while whether the fiber degradation rate in vivo is higher in LF+CE group than the other two (LF and CE) groups need further research. However, it has been reported that the use of an enzyme product in combination with a FAE-producing inoculant did not improve corn silage fermentation or its nutritive value (Lynch, Baah & Beauchemin, 2015). This discrepancy might be caused by the abundantly epiphytic LAB population of corn compared to alfalfa, resulting in inoculated FAE-producing LAB not dominating the epiphytic microbial population at the ensiling process of corn.
The pH value is considered an important indicator reflecting microbial activity and silage fermentation. In this study, all treatments had lower pH values but higher lactic acid content than those of control. The relatively lower pH value and higher content of lactic acid in the CE and LF+CE-treated silage, especially the pH values in LF+CE, declined to around 4.6–4.7, much lower (P<0.05) than that of the addition of CE or LF alone, which could be due to the direct increase of the fermentable substrate through degrading the plant cell wall. All treated silage had a higher ratio of lactic acid and acetic acid, which might indicate that the silage fermentation of alfalfa can be driven towards a homo-fermentative model with the addition of CE and LF.
Additionally, for well-preserved silage, butyric acid content is less than 1.0% DM (McDonald, Henderson & Herson, 1991) and is usually produced by Clostridia. For instance, in sample 1 silage, all treatments, especially LF+CE treatment, had a lower level of butyric acid content (<1.0% DM). Control silage had unsatisfactory preservation due to low content of lactic acid, high pH, and high content of butyric acid and ammonia-N together with high abundance of Clostridium, Anaerosporobacter, and Garciella (Fig. 2C). These bacteria are obligate anaerobes from the class Clostridia. Clostridia is an influential component of bacterial communities and is often detected in grass silages. The appearance of Clostridia is undesirable because they can take advantage of protein and water soluble carbohydrate to produce butyric acid and consequently affect silage quality due to unpleasant odor (Zheng et al., 2017). That may help explain the poor fermentation quality in the control of sample 1, which was consistent with the report by Nishino et al. (2012). However, a similar result was not observed in sample 2 silage, which was possibly because high DM content could efficiently inhibit the growth of Clostridium.
The ammonia-N level reflects the CP degradation in silage, which is an important criterion for evaluating silage quality. Although all additives decreased ammonia-N but increased CP content compared with those of control, especially, the LF+CE treated silage had the highest content of CP and lowest ammonia-N, and similar results were also found in Stylo silage produced using a mixture of LAB and cellulase (Li et al., 2017). The most plausible reason was that the LF+CE addition could efficiently inhibit the growth of undesirable proteolytic bacteria, such as Enterobacter. Enterobacter is generally considered undesirable during the ensiling process because it can ferment lactic acid to acetic acid and other products, thus subsequently causing nutrition loss (Ni et al., 2017). In this study, although Enterobacter was detected in all silage, their relative abundance was much lower in the LF and LF+CE treated silage. In contrast, desirable LAB such as Lactobacillus, Enterococcus, Pediococcus, and Weissella, rod-shaped Lactobacillus plays a critical role in enhancing lactic acid content and reducing pH value (Cai et al., 1998). In the present study, Lactobacillus was the most abundant genus in LF or LF+CE treated silage, which might account for their relatively high fermentation quality.
Conclusion
Compared with addition of CE or LF alone, inoculation of a FAE-producing lactic acid bacteria in combination with cellulase not only improved the silage fermentation quality of alfalfa, but also inhibited the growth of undesirable microbes. Silage inoculants consisting of FAE-producing LAB might be an effective way to improve the silage quality of alfalfa.