Synthesis and biological activity of 1,4-pentadien-3-one derivatives containing triazine scaffolds
- Published
- Accepted
- Received
- Academic Editor
- Giovanna Bosica
- Subject Areas
- Green Chemistry, Natural Products, Organic Chemistry (other), Organic Compounds, Synthetic Organic Chemistry
- Keywords
- 1, 4-pentadien-3-one, Triazine, Antiviral, Antibacterial, Molecular docking studies
- Copyright
- © 2020 Tang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ Organic Chemistry) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. Synthesis and biological activity of 1,4-pentadien-3-one derivatives containing triazine scaffolds. PeerJ Organic Chemistry 2:e3 https://doi.org/10.7717/peerj-ochem.3
Abstract
Background
Literatures revealed that 1,4-pentadien-3-one and triazine derivatives exhibited a wide variety of biological activities. In order to develop highly bioactive molecules, in this study, a series of novel 1,4-pentadien-3-one derivatives containing triazine moieties were synthesized and their antibacterial and antiviral activities were investigated.
Methods
A series of novel 1,4-pentadien-3-one derivatives containing triazine moieties were synthesized and characterized in detail via 1H NMR, 13C NMR and HRMS spectra. The antibacterial activities against Xanthomonas axonopodispv. citri (Xac), Xanthomonas oryzaepv. oryzae (Xoo) and Ralstonia solanacearum (R.s) were evaluated at 100 and 50 µg/mL using a turbidimeter and N. tabacun L. leaves under the same age as that of test subjects. The curative, protective and inactivation activities against tobacco mosaic virus (TMV) at a concentration of 500 µg/mL were evaluated via the half-leaf blight spot method.
Results
The bioassay results showed that some of the target compounds exhibited fine antibacterial activities against Xac and R.s. Particularly, half maximal effective concentration (EC50) values of some target compounds against R.s are visibly better than that of the positive control bismerthiazol (BT). Notably, compound 4a showed excellent inactivation activity against TMV with a EC50 value of 12.5 µg/mL, which was superior to that ofningnanmycin (NNM,13.5 µg/mL). Besides, molecular docking studies for 4awith tobacco mosaic virus coat protein (TMV-CP) showed that the compound was embedded well in the pocket between the two subunits of TMV-CP. These findings indicate that 1,4-pentadien-3-one derivatives containing triazine moieties may be potential antiviral and antibacterial agents.
Introduction
Plant pathogens have become one class of the most serious agricultural problems in the world. They cause threat not only to agricultural products but also to human health (Li et al., 2011; Lorenzo et al., 2017). Plant pathogens diseases, such as citrus canker, rice bacterial leaf blight and tobacco bacterial wilt, were caused by Xanthomonas axonopodispv. citri (Xac), Xanthomonas oryzaepv. oryzae (Xoo) and Ralstonia solanacearum (R.s), respectively. They are difficult to control in agricultural production (Zou, Li & Chen, 2011; Li et al., 2017). In addition, tobacco mosaic virus (TMV) can infect more than 885 plant species, causing nearly $100 million in damage worldwide (Su et al., 2016; Bos, 2000). Therefore, the discovery and development of new antiviral and antibacterial agents with novel action modes are important.
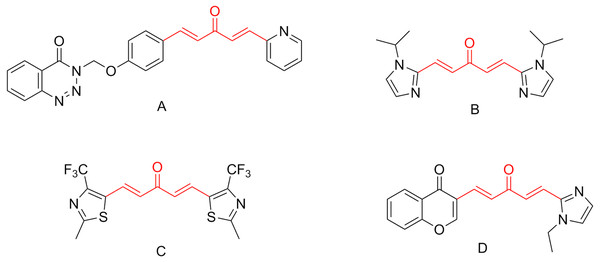
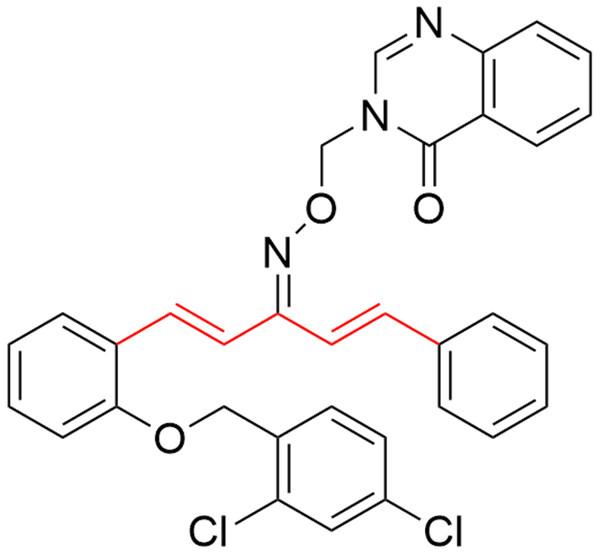
1,4-Pentadien-3-one derivatives, derived from plant metabolic products curcumin, were found to have various biological activities such as antiviral (Zhang et al., 2018), antibacterial (Long et al., 2015), anticancer (Luo et al., 2014), anti-inflammatory (Liu et al., 2014), anti-oxidative (Masuda et al., 2001), and anti-HIV activities (Sharma et al., 2019). Over the past few years, the synthesis and study of pharmacological activity of 1,4-pentadien-3-one derivatives attracted the attention of many chemists (Wang et al., 2017; Zhou et al., 2018). Further study on the structural optimization of 1,4-pentadien-3-one found that introducing benzotriazin-4(3H)-one (Zhang et al., 2018), imidazole (Samaan et al., 2014), thiazole (Wang et al., 2015), or chromone (Chen et al., 2015) moieties (Figs. 1A–1D), could greatly enhance biological activities. Notably, Chen et al. (2019) verified the anti-TMV mechanism of 1,4-pentadien-3-one derivatives (Fig. 2), and found 5-position of 1,4-pentadien-3-one nucleus had played a key role in antiviral activities.
Figure 1: Chemical structures of bioactive molecules bearing 1,4-pentadien-3-one fragment.
Zhang et al. (2018) reported that 1,4-Pentadien-3-onederivatives containing benzotriazin-4 (3H)-one (A), Samaan et al. (2014) reported that 1,4-Pentadien-3-onederivatives containing imidazole (B), Wang et al. (2015) reported that 1,4-Pentadien-3-onederivatives containing thiazole (C), Chen et al. (2015) reported that 1,4-Pentadien- 3-onederivatives containing chromone (D), and tested its biological activities, showing that the introduction of benzotriazin-4(3H)-one, imidazole, thiazole and chromone groups can enhance its biological activities.Figure 2: The anti-TMV mechanism of 1,4-pentadien-3-one derivatives.
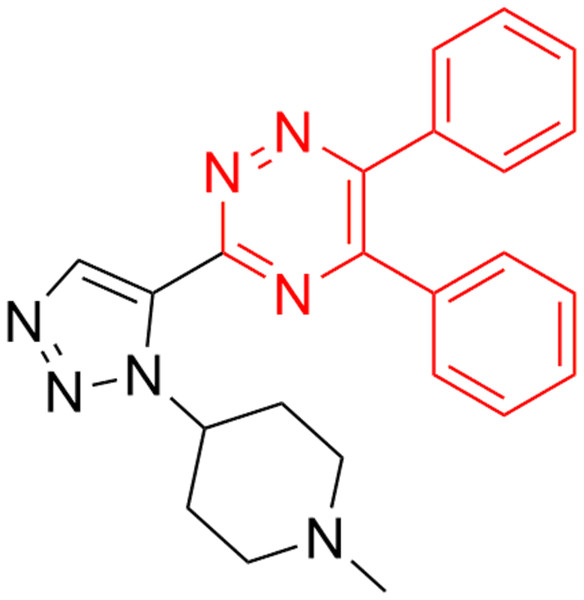
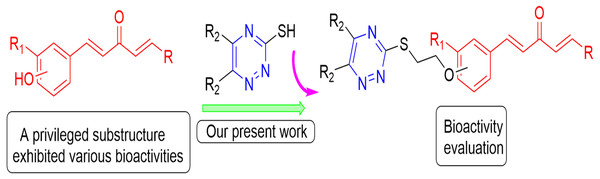
Chen et al. (2019) verified the anti-TMV mechanism of 1,4-pentadien-3-one derivatives.In addition, triazine scaffold has been associated with diversified pharmacological activities (Irannejad et al., 2010), such as antioxidant (Khoshneviszadeh et al., 2016), antithrombotic (Tamboli et al., 2015) antiplatelet (Konno et al., 1993), anticancer (Fu et al., 2017), thromboxane synthetase inhibition (Monge et al., 2010), antimalarial (Ban et al., 2010), α-glucosidase inhibition (Wang et al., 2016), antiviral and antibacterial activities (Tang et al., 2019). Recently, it was found that the heterocyclic nitrogen of the triazine derivatives had tremendous applications in the development of novel agricultural bactericides and virucides (Zhang et al., 2018). Sangshetti & Shinde (2010) reported that the inhibitory effects of triazine and their derivatives against three fungals ((Candida albicans (MIC-25), Aspergillus niger (MIC-12.5) and Cryptococcus neoformans (MIC-25)) are similar to miconazole (Fig. 3). Therefore, triazine group was introduced onto the 5-position of 1,4-pentadien-3-one structure to build a new set of molecules and their biological activities were tested (Fig. 4).
Figure 3: 1,2,4-triazine fragment against three fungals (Candida albicans, Aspergillus niger and Cryp- tococcus neoformans).
Sangshetti & Shinde (2010) reported the potent inhibitory effect of triazine and their derivatives against three fungals ((Candida albicans (MIC-25), Aspergillus niger (MIC-12.5) and Cryptococcus neoformans (MIC-25)) similar to miconazole.Figure 4: Design strategy of title compounds.
Based on the good biological activity of the triazine fragment, the triazine group was introduced into the 5-position of 1,4-pentadien-3-one nucleus to build a new molecular structure, the potency of which was tested in terms of biological activities.Materials & Methods
Instruments and chemicals
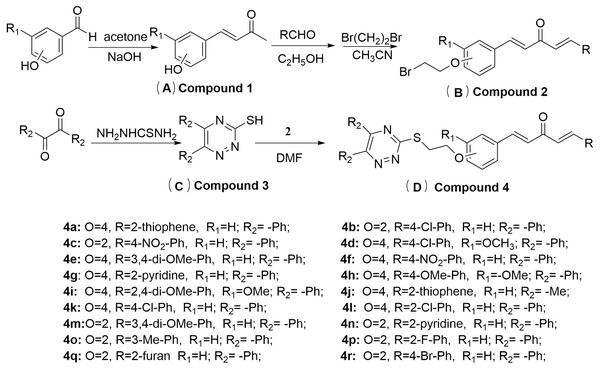
Melting points were determined using an XT-4 digital melting-point apparatus (Beijing Tech. Instrument Co., Beijing, China) and readings were uncorrected. 1H NMR , 13C NMR and 19F NMR spectra were recorded on a 400 MHz spectrometer (Swiss Bruker, Fällanden, Switzerland) with DMSO and CDCl3 as the solvent and tetramethylsilane as the internal standard. The course of the reaction was monitored by thin-layer-chromatography analysis on silica gel GF254 (Qingdao Haiyang Chemical Company, Ltd., Qingdao, China), and spots were visualized with ultraviolet (UV) light. High-resolution mass spectrometry (HRMS) was conducted by using a Thermo Scientific Q Exactive (Thermo Scientific, Missouri, USA). The molecular docking was performed by using DS-CDocker implemented in Discovery Studio (version 4.5). All reagents and solvents were purchased from Chinese Chemical Reagent Company and were of analytical grade reagents. The synthetic route to1,4-pentadien-3-one derivatives containing triazine moiety was shown in Fig. 5.
Figure 5: Synthesis route for the target compounds.
(A) Compound 1, (B) Compound 2, (C) Compound 3, (D) Compound 4.General procedure for the synthesis of intermediates
A synthetic route to 1,4-pentadien-3-one derivatives containing a triazine moiety was designed and shown in Fig. 5. According to previously reported methods (Chen et al., 2019; Tang et al., 2019; Gan et al., 2017), intermediates 1 and 2 could be obtained. Using benzyl, biacetyl and thio-semicarbazide as the initial materials in acetic acid and water was stirred at 100−110 °C for 6–8 h to obtain the intermediate 3 (Tang et al., 2019).
General procedure for the synthesis of target compounds 4a-4r
Reaction mixture was added to a solution of intermediate 2 (12 mmol), intermediate 3
(10 mmol) and K2CO3 (30 mmol) in dimethylformamide and stirred at room temperature for
6–8 h. After the reaction was completed (monitored by TLC), the mixture was extracted with ethyl acetate (30 mL × 3). The solvent was removed under reduced pressure. Residue was purified by silica-gel column chromatography using petroleum ether/ethyl acetate (3:1 v/v) to obtain target compounds 4a–4r. The 1H NMR, 13C NMR, 19F NMR and HMRS spectra of the target compounds 4a–4r are also provided in the Supplemental Information.
Bioactivity assay
Antibacterial activity in vitro
The antibacterial activities of the title compounds against Xanthomonas axonopodispv. citri (Xac), Xanthomonas oryzaepv. oryzae (Xoo) and Ralstonia solanacearum (R.s) were evaluated at 100 µg/mL using a turbidimeter (Tang et al., 2019; Zhang et al., 2018). This test method is provided in the Supplemental Information.
Antiviral activities in vivo
Using N. tabacun L. leaves under the same age as that of test subjects, the curative, protective and inactivation activities against TMV (in vivo) at a concentration of 500 µg/mL were evaluated by the half-leaf blight spot method (Chen et al., 2019).This test method is provided in the Supplemental Information.
Molecular docking
The molecular docking was performed by using DS-CDocker implemented in Discovery Studio (version 4.5). This test method is provided in the Supplemental Information.
Results
Antibacterial activities in vitro
The antibacterial activities of target compounds have been evaluated by the turbidimeter test (Zhang et al., 2018; Tang et al., 2019). Results in Table 1 indicated that some of synthesized compounds exhibited appreciable antibacterial activities against Xoo, R.s and Xac at the concentrates of 100 µg/mL. Among these derivatives, 4n and 4p exhibited excellent bactericidal effect against Xoo, with inhibition rates of 60.5% and 56.5%, respectively, which were superior to bismerthiazol (BT, 56.1%). In addition, as demonstrated in Table 1, the designed compounds displayed certain bactericidal effect toward R.s. Studies on the inhibition effect of title compounds suggested that 4a, 4b, 4j and 4k exhibited excellent inhibition effect against R.s with the inhibition rates of 58.2, 53.9, 53.5 and 61.9%, respectively, which were better than that of BT (52.1%). We also noticed that compounds 4k (91.8%) and 4l (95.4%) exposed better antibacterial activity toward Xac than that of BT (70.5%).
| Compd. | Inhibition (%) | |||||
|---|---|---|---|---|---|---|
| Xoo | R.s | Xac | ||||
| 100 µg/mL | 50 µg/mL | 100 µg/mL | 50 µg/mL | 100 µg/mL | 50 µg/mL | |
| 4a | 19.7 ± 4.3 | 18.9 ± 3.5 | 58.2 ± 2.4 | 58.2 ± 3.7 | 43.7 ± 2.2 | 37.6 ± 2.4 |
| 4b | 48.5 ± 5.2 | 33.5 ± 3.0 | 53.9 ± 6.5 | 44.6 ± 1.8 | 56.5 ± 1.1 | 41.4 ± 1.3 |
| 4c | 20.9 ± 6.5 | 10.6 ± 1.8 | 15.7 ± 9.9 | – | 42.3 ± 2.1 | 44.8 ± 1.8 |
| 4d | 13.2 ± 6.3 | 12.7 ± 2.9 | 38.0 ± 3.3 | 37.0 ± 4.3 | 38.2 ± 3.7 | 33.5 ± 3.6 |
| 4e | 54.6 ± 1.8 | 45.0 ± 2.9 | 37.6 ± 4.3 | 28.0 ± 2.1 | 30.8 ± 1.0 | 37.2 ± 1.5 |
| 4f | 12.3 ± 1.2 | 3.9 ± 7.5 | 28.5 ± 7.5 | 23.0 ± 3.2 | 47.4 ± 2.2 | 35.1 ± 2.7 |
| 4g | 43.8 ± 2.7 | 43.8 ± 2.3 | 28.5 ± 3.1 | 22.6 ± 2.6 | 35.9 ± 13.7 | 29.1 ± 3.9 |
| 4h | 13.9 ± 4.5 | 30.7 ± 6.6 | 28.2 ± 2.6 | – | 41.7 ± 4.4 | 43.3 ± 0.8 |
| 4i | 55.6 ± 0.9 | 54.4 ± 2.8 | 18.0 ± 2.9 | 28.6 ± 1.3 | 61.6 ± 8.8 | 43.4 ± 2.2 |
| 4j | 48.6 ± 1.1 | 38.9 ± 2.4 | 53.5 ± 2.9 | 45.0 ± 5.5 | 64.8 ± 2.9 | 43.0 ± 9.3 |
| 4k | 10.5 ± 4.7 | 5.9 ± 3.7 | 61.9 ± 2.7 | 49.2 ± 2.5 | 91.8 ± 2.3 | 85.6 ± 4.7 |
| 4l | 14.1 ± 2.3 | 21.2 ± 4.8 | 45.3 ± 4.4 | 28.6 ± 2.5 | 95.4 ± 9.0 | 68.1 ± 7.9 |
| 4m | 43.6 ± 3.0 | 28.5 ± 4.2 | 18.2 ± 1.8 | 17.0 ± 3.7 | 41.2 ± 3.9 | 32.5 ± 5.1 |
| 4n | 60.5 ± 0.9 | 44.3 ± 7.5 | 44.6 ± 8.7 | 32.0 ± 8.7 | 35.4 ± 1.3 | 32.3 ± 2.5 |
| 4o | 41.8 ± 7.4 | 25.1 ± 3.0 | 43.5 ± 4.4 | 37.1 ± 3.4 | 41.0 ± 4.4 | 32.0 ± 7.6 |
| 4p | 56.5 ± 3.9 | 27.6 ± 3.9 | 21.3 ± 6.2 | 12.7 ± 9.6 | 41.1 ± 1.5 | 28.4 ± 2.7 |
| 4q | 24.0 ± 9.9 | 20.2 ± 2.4 | 18.9 ± 1.8 | 16.4 ± 1.8 | 74.6 ± 1.8 | 50.0 ± 2.2 |
| 4r | 15.1 ± 4.8 | 11.0 ± 9.0 | 24.4 ± 7.6 | 11.3 ± 8.0 | 51.8 ± 4.4 | 14.8 ± 2.4 |
| BTb | 56.1 ± 7.3 | 49.3 ± 5.4 | 52.1 ± 3.4 | 44.2 ± 3.9 | 70.5 ± 1.5 | 33.6 ± 1.7 |
To further understand the antibacterial activity of the title compounds, the EC50 values of some title compounds were calculated and summarized in Table 2. Notably, compounds 4a, 4b, 4j and 4k exhibited good inhibition effects against R.s, with half maximal effective concentration (EC50) values of ranging from 0.43–4.76 µg/mL, which were better than that of BT (EC50 = 49.5 µg/mL). Meanwhile, compounds 4j and 4k showed remarkable antibacterial activities against Xac with the EC50 values of 55.53 and 129.1 µg/mL, which were better than that of BT (EC50 = 153.7 µg/mL).
| Tested bacterias | Compd. | Regression equation | r2 | EC50 (µg/mL) |
|---|---|---|---|---|
| Xoo | 4e | y = 0.4750x + 4.0608 | 0.9526 | 94.9 |
| BTb | y = 1.5696x + 1.8988 | 0.9551 | 94.6 | |
| Xac | 4j | y = 0.9367x + 3.3659 | 0.9509 | 55.5 |
| 4k | y = 0.6755x + 3.5689 | 0.9181 | 129.1 | |
| BTb | y = 0.3926x + 4.1415 | 0.9072 | 153.7 | |
| R.s | 4a | y = 1.0922x + 4.2593 | 0.9619 | 4.76 |
| 4b | y = 0.4261x + 5.1569 | 0.9107 | 0.4 | |
| 4j | y = 0.6032x + 4.8698 | 0.9116 | 1.6 | |
| 4k | y = 0.7208x + 4.8188 | 0.9303 | 1.8 | |
| BTb | y = 1.0223x + 3.2674 | 0.9095 | 49.5 |
Antiviral activities against TMV in vivo
The antiviral activities of the title compounds 4a–4r against tobacco mosaic virus (TMV) were evaluated by the half leaf method (Chen et al., 2019) and the results were summarized in Table 3 and Fig. 6. It was found that some of the title compounds exhibited good antiviral activity against TMV in vivo. Compounds 4f, 4k and 4l showed remarkable curative activity against TMV, with values of 53.8, 66.3 and 59.9%, respectively. Which were better than that of ningnanmycin (NNM, 45.7%). Meanwhile, compound 4 h (61.4%) exhibited excellent protection activity, also superior to NNM (53.4%). Overall, most of the compounds indicated general inactivation activity against TMV at 500 µg/mL.
| Compd. | Curative activity (%) | Protective activity (%) | Inactivation activity (%) |
|---|---|---|---|
| 4a | 30.1 ± 0.21 | 16.1 ± 0.32 | 66.2 ± 0.02 |
| 4b | 44.4 ± 0.05 | 54.5 ± 0.03 | 48.2 ± 0.02 |
| 4c | 40.1 ± 0.05 | 35.5 ± 0.12 | 57.1 ± 0.02 |
| 4d | 29.3 ± 0.07 | 53.9 ± 0.02 | 63.6 ± 0.03 |
| 4e | 44.1 ± 0.03 | 14.9 ± 0.15 | 39.4 ± 0.07 |
| 4f | 53.8 ± 0.07 | 39.5 ± 0.02 | 50.1 ± 0.02 |
| 4g | 44.5 ± 0.03 | 44.4 ± 0.11 | 44.9 ± 0.07 |
| 4h | 47.6 ± 0.07 | 61.4 ± 0.04 | 53.5 ± 0.05 |
| 4i | 27.3 ± 0.04 | 33.5 ± 0.11 | 24.3 ± 0.07 |
| 4j | 43.1 ± 0.02 | 26.7 ± 0.03 | 24.1 ± 0.11 |
| 4k | 66.3 ± 0.01 | 24.1 ± 0.28 | 27.7 ± 0.01 |
| 4l | 59.9 ± 0.07 | 18.4 ± 0.02 | 31.9 ± 0.09 |
| 4m | 48.8 ± 0.06 | 28.6 ± 0.17 | 22.3 ± 0.09 |
| 4n | 37.4 ± 0.05 | 27.5 ± 0.19 | 27.1 ± 0.07 |
| 4o | 39.5 ± 0.02 | 22.4 ± 0.08 | 57.7 ± 0.01 |
| 4p | 46.6 ± 0.08 | 41.2 ± 0.08 | 28.2 ± 0.09 |
| 4q | 38.5 ± 0.01 | 31.6 ± 0.01 | 33.5 ± 0.02 |
| 4r | 42.4 ± 0.02 | 34.1 ± 0.11 | 33.5 ± 0.02 |
| NNMb | 45.7 ± 2.61 | 53.4 ± 2.42 | 77.3 ± 1.60 |
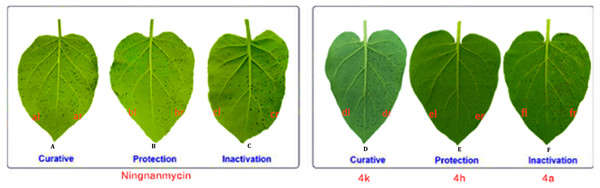
Figure 6: Tobacco leaf morphology effects of the NNM and 4k, 4h and 4a against TMV in vivo.
(A) The curative activity of the ningnanmycin (NNM). The left side of the leaf (al) was smeared with compound NNM, and the right side of the leaf (ar) was not treated with compound NNM. (B) The protective activity of the ningnanmycin. The left side of the leaf (bl) was smeared with compound NNM, and the right side of the leaf (br) was not treated with compound NNM. (C) The inactivation activity of the ningnanmycin. The left side of the leaf (cl) indicated that smeared with compound NNM, and the right side of the leaf (cr) indicated that not treated with compound NNM. (D) The curative activity of the compound 4k. The left side of the leaf (dl) was smeared with compound 4k, and the right side of the leaf (dr) was not treated with compound 4k. (E) The protective activity of the compound 4h. The left side of the leaf (el) was smeared with compound 4h, and the right side of the leaf (er) was not treated with compound 4h. (F) The inactivation activity of the compound 4a. The left side of the leaf (fl) was smeared with compound 4a, and the right side of the leaf (fr) was not treated with compound 4a.Based on the previous bioassays, the EC50 values of some title compounds were tested and are listed in Table 4. Compound 4a exhibited excellent inactivation activity against TMV, with the EC50 values of 12.5 µg/mL, which was better than that of NNM (EC50 = 13.5 µg/mL). Moreover, compounds 4k and 4l exhibited the preferably curative activity against TMV, with EC50 values of 11.5 and 12.1 µg/mL, respectively, which were superior to that of NNM (EC50 = 82.2 µg/mL).
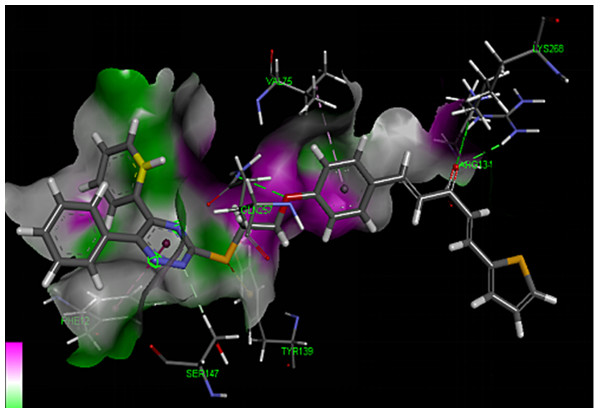
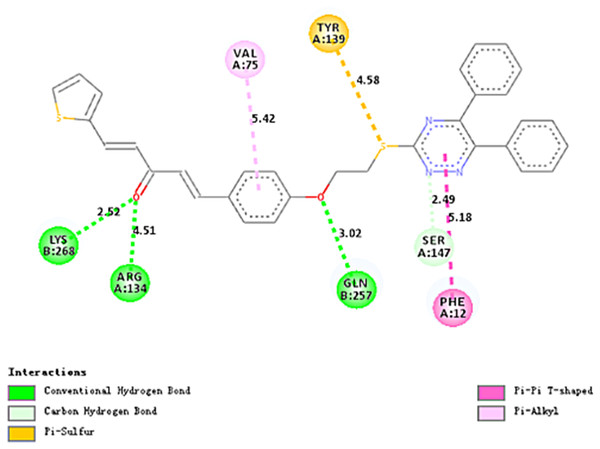
Molecular docking studies
Molecular docking studies (Figs. 7 and 8) for 4a with tobacco mosaic virus coat protein (TMV-CP) (PDB code:1EI7). Molecular docking results revealed that compound 4a was the most preferred compound based on the analysis followed by 4d and so on (Table 3). Compound 4a binding orientation clearly is described by Figs. 7 and 8, it forms one hydrogen bond with PHEA:12 with highest docking score (2.49 Å) among the designed molecules and the glide energy was also less compared to others showing few hydrophobic interactions with specific residues like as TYRA:139, VALA:75, LYSB:268 etc.
Discussion
Structure–activity relationships of antibacterial activities
The antibacterial results in Tables 1 and 2 also indicated that the different groups on R had significant effects on the antibacterial activities of the title compounds. Obviously, the presence of a C6H4Cl group can effectively enhance the antibacterial activity against Xac. For example, the compounds 4k and 4l, which contain R = 4-Cl- C6H4 and R = 2-Cl- C6H4 groups respectively, exhibited EC50 values of 55.53 and 129.1 µg/mL, which were better than that of BT (EC50 = 153.7 µg/mL). Meanwhile, when R was substituted with thiophene-2-yl and 4-Cl- C6H4 groups, the corresponding compounds 4a, 4b, 4j and 4k exhibit remarkable antibacterial activities against R.s, with the EC50 values of ranging from 0.43–4.76 µg/mL, which were better than that of BT (EC50 = 49.5 µg/mL).
| Compd. | against TMV | regression equation | r2 | EC50 |
|---|---|---|---|---|
| 4a | Inactivation activity | y = 0.6712x + 4.2637 | 0.9234 | 12.5 |
| 4d | Inactivation activity | y = 0.8253x + 3.7000 | 0.9279 | 37.6 |
| 4h | Protection activity | y = 0.4739x + 4.2865 | 0.9833 | 32.1 |
| 4k | Curative activity | y = 0.4261x + 4.5479 | 0.9382 | 11.5 |
| 4l | Curative activity | y = 0.6542x + 4.2925 | 0.9191 | 12.1 |
| NNMb | Curative activity | y = 0.4415x + 4.1563 | 0.9720 | 81.4 |
| Protection activity | y = 0.4732x + 4.0939 | 0.9097 | 82.2 | |
| Inactivation activity | y = 0.8498x + 4.0381 | 0.9702 | 13.5 |
Figure 7: Three dimensional diagrams of compound 4a docked with TMV-CP.
Figure 8: Two-dimensional diagrams of compound 4a docked with TMV-CP.
The two-dimensional diagram contains conventional hydrogen bonds, carbon–hydrogen bonds, Pi-Pi T-shaped bonds and Pi-Alkyl bonds.Structure–activity relationships of antiviral activities
The antiviral bioassay results indicated that the title compounds showed excellent antiviral activity against TMV. The preliminary SAR results were dropped based on the anti-TMV activity (as shown in Tables 3 and 4). The results indicated that when R was the 4-NO2- C6H4 (4f), 4-Cl- C6H4 (4k) or 2-Cl- C6H4 (4l) group, the corresponding title compounds exhibited good curative activities. Furthermore, when the R was 4-OMe- C6H4 group, the protective activity of corresponding compound 4h was better than that of NNM (EC50 = 82.2 µg/mL), with an EC50 values of 32.1 µg/mL.
Conclusions
In short, a series of 1,4-pentadien-3-one derivatives containing triazine scaffolds were synthesized. The obtained bioassay results revealed that some of the title compounds exhibited excellent antibacterial or antiviral activities that were better than the commercial agents. In particular, compound 4a showed prominent inactivation activity against TMV. Furthermore, compound 4a had strong binding capability with TMV-CP. These results proved that the 1,4-pentadien-3-one derivatives containing triazine scaffolds possessed antiviral and antibacterial activities.
Supplemental Information
Spectral data and biological test methods for all compounds
Three groups of initial screening values inhibition effect of the title compounds against R.s
The labels are 1, 2, and 3, and the experiment is repeated three times at 100 µg/mL and 50 µg/mL, CK is a blank control, BT is a commercial drug, and the inhibition rate is calculated by a formula to obtain an average value of three primary sieves, but the calculation is performed. Taking R.S as an example, other plant pathogen-like calculation methods are not provided. and the ”-” represents a negative or zero inhibition rate.